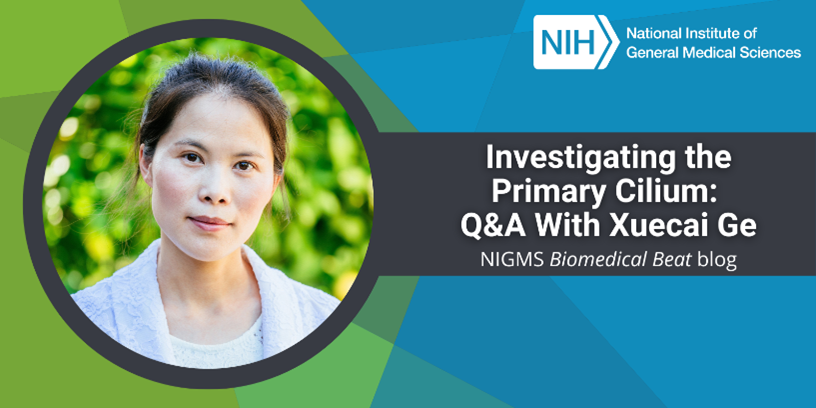
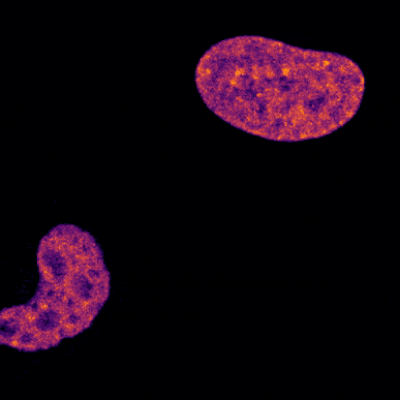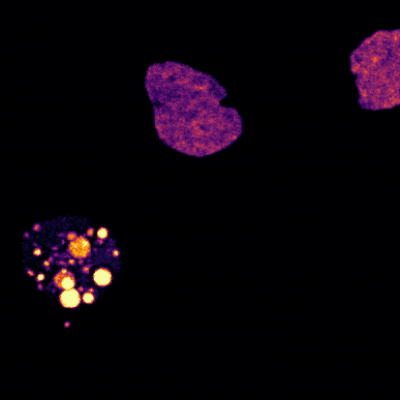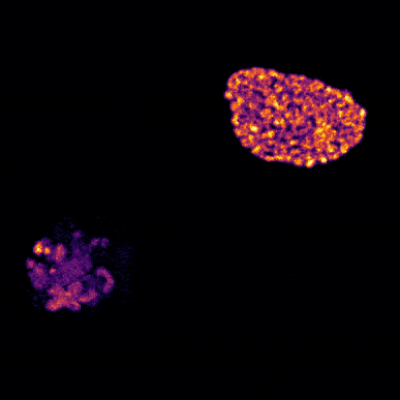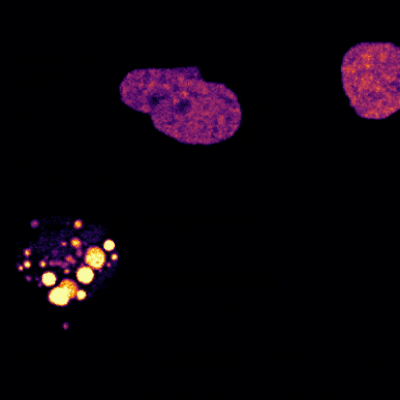
The brain is a large and complex organ, but some very small structures guide its development. Xuecai Ge, Ph.D., an associate professor of molecular and cell biology at the University of California, Merced (UC Merced), has devoted her career to understanding one of these structures called the primary cilium. In an interview, Dr. Ge shared how her childhood experience inspired her to study science and what makes the primary cilium fascinating.
Q: How did you first become interested in science?
A: When I was a little kid, my mom was a primary care doctor, and I saw her treat patients in our community. I noticed that no matter who got a particular illness, she could use the same medicine to treat them. My little mind was amazed that the same medicine could work for so many different people! I think this early experience planted the original seed of my interest in life science.
Continue reading “Investigating the Primary Cilium: Q&A With Xuecai Ge”