I can still remember that giddy feeling I had seven years ago, when I first read about the “zombie ant.” The story was gruesome and fascinating, and it was everywhere. Even friends and family who aren’t so interested in science knew the basics: in a tropical forest somewhere there’s a fungus that infects an ant and somehow takes control of the ant’s brain, forcing it to leave its colony, crawl up a big leaf, bite down and wait for the sweet relief of death. A grotesque stalk then sprouts from the poor creature’s head, from which fungal spores rain down to infect a new batch of ants.

The problem is, it doesn’t happen quite like that. David Hughes, the Penn State University entomologist who reported his extensive field observations of the fungus/ant interactions in BMC Ecology ![]() , which caused much excitement back in 2011, has continued to study the fungus, Ophiocordyceps unliateralis, and its carpenter ant host, Camponotus leonardi.
, which caused much excitement back in 2011, has continued to study the fungus, Ophiocordyceps unliateralis, and its carpenter ant host, Camponotus leonardi.
In late 2017, Hughes and his colleagues published an article in PNAS ![]() in which they used sophisticated microscopy and image-processing techniques to describe in great detail how the fungus invades various parts of the ant’s body including muscles in its legs and head.
in which they used sophisticated microscopy and image-processing techniques to describe in great detail how the fungus invades various parts of the ant’s body including muscles in its legs and head.
Although Hughes’s earlier BMC Ecology paper showed fungus in the head of an ant, the new study reveals that the fungus never actually enters the brain.
To me, the new finding somehow made the fungus’ control over the ant even more baffling. What exactly was going on?
To find out, I spoke with Hughes and his graduate student Maridel Fredericksen.

Chris Palmer:
How did you first encounter the fungus O. unilateralis?
David Hughes:
I was doing my PhD on a group of parasites called Strepsiptera that control ant and wasp behavior. They’re very interesting, and they do cool things just like this fungus. But they’re not amenable to experimentation: they don’t infect their hosts in the lab, and you can’t look at their genes or the chemicals they produce.
Then I read about Ophiocordyceps unliateralis in a dusty old publication. So, I made a trek to a rainforest in southern Thailand to observe them, probably 12 years ago now, and it’s been fun ever since.
Chris Palmer:
What does the fungus do to the carpenter ant?
David Hughes:
This is a highly specialized fungal parasite that enters the body of a foraging ant. Over the course of a couple weeks the fungus grows within the ant’s body from a small clump of cells to a large interconnected group of cells that cooperate and communicate with each other.
It’s at this stage that the fungus manipulates the ant’s behavior to leave the colony. Ants are extremely good at keeping the interior of their colony clean. If the fungus were to kill its ant inside the colony and try to grow from its dead body, which is a necessary part of the lifecycle, it would not be successful because fellow ants would dispose of the body before the fungus could sprout. Instead, the fungus manipulates the ant to go die outside of the colony.
Chris Palmer:
What else have you learned about this fungus in the years since your initial observations?
David Hughes:
We knew nothing about this system, even though it had been first recorded in 1859 by Alfred Russel Wallace, the co-discoverer of natural selection along with Charles Darwin. So, our early work was descriptive natural history. A few years later, we studied the ant’s genes and the chemicals the fungus produced. We also conducted field geographic studies aimed at understanding where and when the fungus evolved. We learned that a wide range of insects—from caterpillars to moths to beetles—are infected by this group of fungi. But it’s when the fungus infects ants and wasps that it manipulates behavior so gloriously.
Chris Palmer:
What motivated the new study?
Maridel Fredericksen:
As David mentioned, we knew a lot about the behavior of the ants. We also had the fungus genome and knew the chemicals the fungus secretes to manipulate the ant. But we didn’t really have anything in between, at the cellular level, which is the level at which the fungus itself behaves. Specifically, we wanted to look more closely at how the fungal cells were interacting with the ant’s muscle cells and, potentially, its brain.
Chris Palmer:
What did you find?
David Hughes:
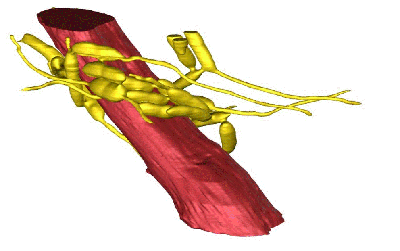
We saw something rather special—a three-dimensional network of interconnected fungal cells surrounding muscle fibers in both the leg and the jaw. Some of those fungal cells had hyphae, which is the exploratory part of a fungus, entering into the muscles. From this, we concluded that the networks of fungal cells are used for communicating and coordinating a collective action. In this case, we suspect the hyphae feed from the energy-rich muscle and pass food down through the network.
Chris Palmer:
Were you surprised to learn that the fungus wasn’t in fact invading the brain?
David Hughes:
We had always suspected this. Earlier studies showed it was in the head, but the anatomical images didn’t have the spatial resolution to conclude that it was definitely in the brain. In retrospect, as is often the case in scientific discoveries, you would say, “Of course, it doesn’t need to go to the brain because it’s focused on controlling the muscle.” It’s one of those things that becomes very clear in hindsight.
Chris Palmer:
Do you know how the fungus is controlling the muscles?
David Hughes:
Two things are required for muscle movement: a signal from the nervous system and muscle fibers that contract. In the infected ant the fungus is forming this sprawling three-dimensional collective unit around the muscle. We suspect that the fungus produces chemicals that affect muscle contraction.
This is extremely interesting when one thinks of the wide variety of human diseases, including spinal cord injuries and carpal tunnel syndrome, where muscles break down following a disruption to nervous-system input.
In infected ants, the muscles don’t die off. Instead, they begin to be controlled by the fungus, rather than the nervous system. We suspect that the fungus starts producing chemicals that cause muscle contractions, and they do this at a set rate. This enables the fungus to make the ant move from point A to point B—in this case from the center of the colony to outside the colony and up on a twig to drop down spores on a new batch of ants.
Chris Palmer:
In your latest paper, you use a technique called serial block-face scanning electron microscopy combined with an automated image-processing algorithm. Can you describe that technique and how you used it to arrive at your conclusions?
Maridel Fredericksen:
We first collected the ants and the fungus in South Carolina. So, yeah, this odd behavior occurs in the U.S., too. It’s not just an exotic, tropical thing. We then took a few small tissue samples from the leg muscles and jaw muscles of infected ants. A technician places the tissue inside the microscope, which automatically cuts each tissue into nanometer-thick slices using a diamond knife. The result is thousands of slices per micrometer (one thousandth of a millimeter). Our co-author and microscopy expert, Missy Hazen, then took high-resolution photographs of each slice using an electron microscope. The whole process took about 24 hours per sample.
Chris Palmer:
How did you combine all those images into something useful?
Maridel Fredericksen:
We’d get stacks of images and I would align them to reconstruct 3-D images. At first, I worked on one muscle fiber with the fungus wrapped around it. It took a long time—about a month—to reconstruct, even though it was a really, really small part of one stack of images. For each image, I used a computer program to draw around a muscle fiber and the surrounding fungal cells. Then I did that for each of the one thousand images that were in the stack. It was a pretty tedious process.
But then we collaborated with Danny Chen and Yizhe Zang from Notre Dame, and thankfully they were able to automate the process with their image-processing algorithms.
Chris Palmer:
How does the algorithm work?
Maridel Fredericksen:
We would all look at images and say, “Okay, this is muscle, this is fungus.” We did that for just two images. They used those marked-up images to train their algorithm, which then differentiated fungus from muscle for the thousands of remaining images.
Chris Palmer:
And did you use the same procedure to look at the brain?
Maridel Fredericksen:
No. We used a different microscopy technique to examine the brain. That technique revealed that the fungal hyphae bodies did not penetrate the brain.
Chris Palmer:
How much time do you think the algorithm saved compared to doing it by hand?
Maridel Fredericksen:
Literally years. It took them a while to develop their model, but afterwards everything went super fast.
Chris Palmer:
So, the fungus is controlling the leg muscles directly. But is it also controlling the jaw muscles to produce the biting down behavior that anchors the ant to the leaf?

David Hughes:
Yes, right, except for that last bit about the biting. We have no idea how that happens, but we think the brain is involved. It’s the magical element of the whole thing, which will take quite a number of years to figure out, I suppose.
Chris Palmer:
What’s your plan for learning how the biting works?
David Hughes:
We’re working on a rather nice technique that can scan across the brain on a micron-by-micron scale to measure the chemical composition of tissue inside the brain. Because, while we didn’t find the fungus inside the brain, we strongly suspect based upon some initial images that there are far more protein receptors for the neurotransmitter serotonin in the brains of infected ants. Perhaps the increase in serotonin receptors, which can result in significant changes to behavior, is driven by chemicals released by the fungus.
Chris Palmer:
What ultimately do you hope a full understanding of this fungus can bring to science and human health?
David Hughes:
The micron-level understanding of a parasitic microbe in a body is really advanced in this system compared to other systems. So, it’s easier and faster to study fungal infections in ants than in humans.
Also, there is a lot of research on human diseases caused by bacteria and viruses. We’ve largely ignored fungal diseases, which, as a group, kill more humans every year—more than 1.3 million—than malaria. But we don’t really have anything in our arsenal to kill them.
Chris Palmer:
Why are fungal diseases so hard to treat?
David Hughes:
A lot of people will be surprised to hear this, but fungi are more related to animals than they are to plants. Because of this relationship, virtually everything that we throw at a fungus to kill it is going to kill human cells just as quickly.
Our work provides a unique insight into how fungi communicate and coordinate inside an animal’s body, which could help in designing drugs to target fungi. For example, if it turns out that it’s critical that they form these interconnected tubes from one cell to another, then perhaps we can derive something that stops tube formation and ultimately thwarts infection.

