You may know that antioxidants can help protect your cells from oxidative damage, but do you know about selenium—an element often found in special proteins called antioxidant enzymes? Selenium is essential to your body, which means you must get it from the food you eat. But it’s a trace element so you only need a small amount to benefit from its effects. In addition to its antioxidant properties, it’s also important for reproduction, DNA synthesis, and hormone metabolism.
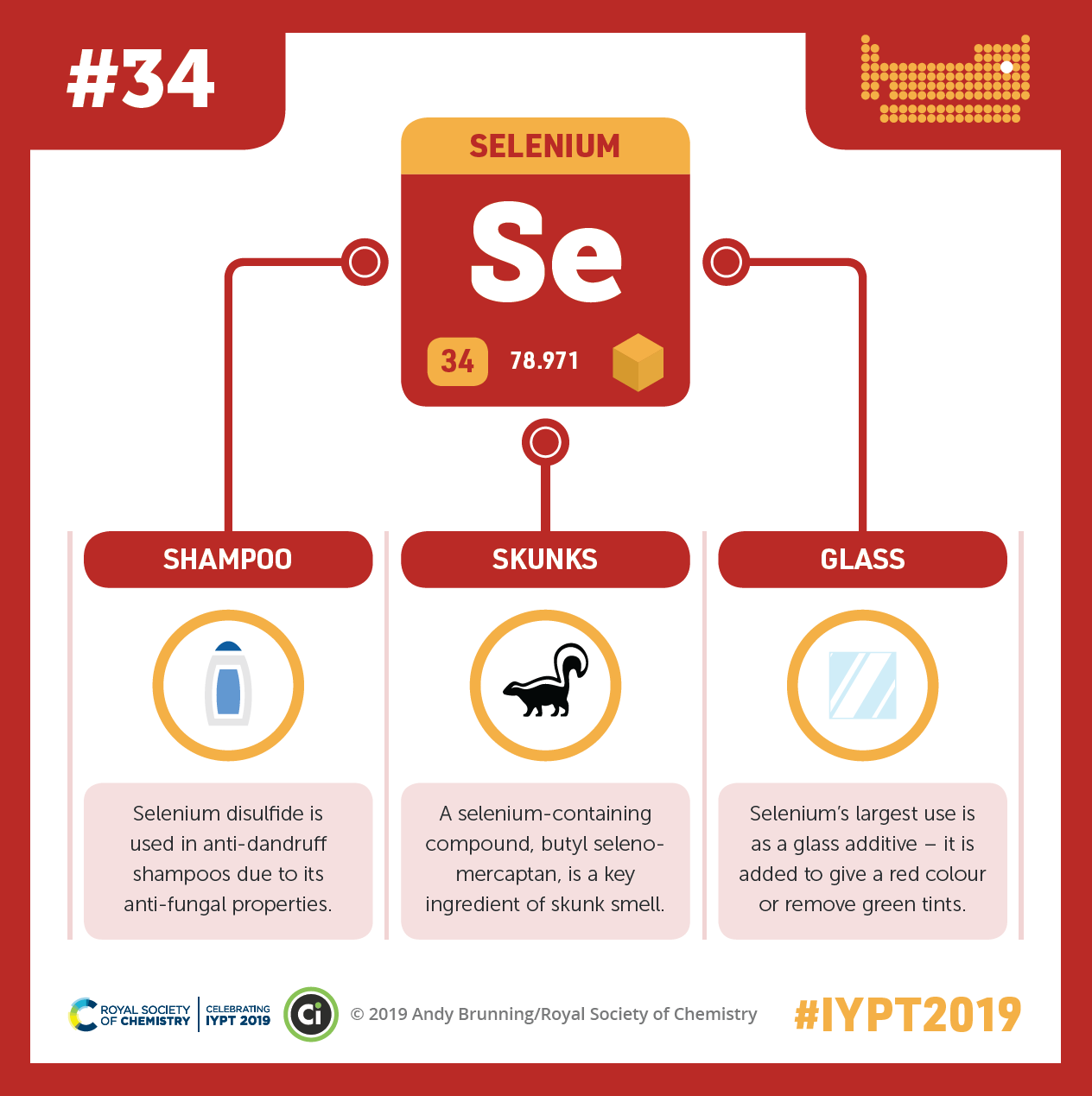 In our bodies, selenium works in antioxidant enzymes to help protect us from oxidative damage. The element is also found in antidandruff shampoo, the smelly spray of skunks, and glass. Credit: Compound Interest. CC BY-NC-ND 4.0. Click to enlarge
In our bodies, selenium works in antioxidant enzymes to help protect us from oxidative damage. The element is also found in antidandruff shampoo, the smelly spray of skunks, and glass. Credit: Compound Interest. CC BY-NC-ND 4.0. Click to enlarge
The Selenium Sweet-Spot
Being that selenium is an essential trace element, our bodies don’t need much of it. But they do need some, which we receive from our diets. Amounts that are too high or too low can lead to health problems. Too much selenium can cause effects like nausea, diarrhea, and skin rashes. Extremely high amounts can lead to more severe effects like difficulty breathing, heart attack, and kidney and heart failure.
People who don’t get enough selenium can develop a specific type of heart disease or arthritis, and men with selenium deficiency can develop infertility. Scientists have found some links between selenium deficiency and cancer, heart disease, and cognitive decline, as well as thyroid disease. But they need more research to fully understand the element’s role in these diseases.
Sources of Selenium
The amount of selenium in foods depends on the soil they grew from—or for animals, the soil of its food. North American soil is selenium rich, so deficiencies in Americans are rare, but people from some parts of China, Russia, and Europe, where the soil has low selenium levels, may need to supplement their diets.

Many foods provide the amounts of selenium that we need. In fact, just one Brazil nut has more than the daily recommended amount! Other common foods that contain the trace element include:
- Seafood
- Meat, eggs, and dairy
- Breads, cereals, and other grains
Selenium in Proteins
Selenium is a close chemical relative to sulfur, and the two sulfur-containing amino acids, methionine and cysteine, can be made using selenium in sulfur’s place—resulting in selenomethionine and selenocysteine, respectively. Selenomethionine can replace methionine in proteins without affecting their function, and it may even serve as a storage reservoir ensuring that the body has access to selenium when it needs it.
Incorporation of selenocysteine into proteins—called selenoproteins—is genetically encoded, requiring specific cellular machinery. At least 25 selenoproteins are encoded in our genome and are synthesized as other proteins are—DNA is transcribed into mRNA, which is translated into protein. The specific sequence of the mRNA alerts the cell’s machinery that selenocysteine needs to be used and recruits a tRNA molecule, whose only job is to insert selenocysteine into proteins.
A number of selenoproteins act as antioxidant enzymes, preventing potentially dangerous reactive oxygen species from damaging cells. Some selenoproteins catalyze the conversion of hydrogen peroxide—an oxidant often found in cells—to water. The antioxidant function of these enzymes relies on selenium’s presence in its active site. Other selenoproteins function to:
- Protect cells from oxidative damage in other ways
- Store and transport selenium between tissues
- Catalyze the synthesis of the specialized selenium tRNA
- Serve other roles that are still being investigated—including some anticancer and immune response mechanisms
NIGMS-Supported Selenium Research
NIGMS supports selenium-related research, and some of these scientists are:
- Investigating how dietary selenium affects protein expression and synthesis
- Using research organisms to identify functions of certain selenium-containing proteins
- Developing methods to specifically incorporate selenium into proteins using the cell’s own machinery, which may become more useful for designing proteins with other unnatural amino acids in them
- Studying how selenium is recycled and reused
Check out our other posts on elements.