
“I wanted to give up so many times. Although I tried to remain positive, I never thought I’d be able to finish my Ph.D. But I made it, and I’m extremely proud of myself,” says Amie Fornah Sankoh, Ph.D., a research scientist with Dow Chemical Company who received NIGMS support as a graduate student.
Human and Plant Communication
Dr. Sankoh has loved science and mathematics since she was just a child growing up in Sierra Leone. When she was 3 years old, Dr. Sankoh became deaf from a childhood disease. Math, unlike other subjects, is very visual, which played a part in her interest in it. “Before I learned American Sign Language when I was 15 years old, I could only understand one language: mathematics,” Dr. Sankoh says.
Continue reading “Amie Fornah Sankoh Achieves a Scientific Dream”







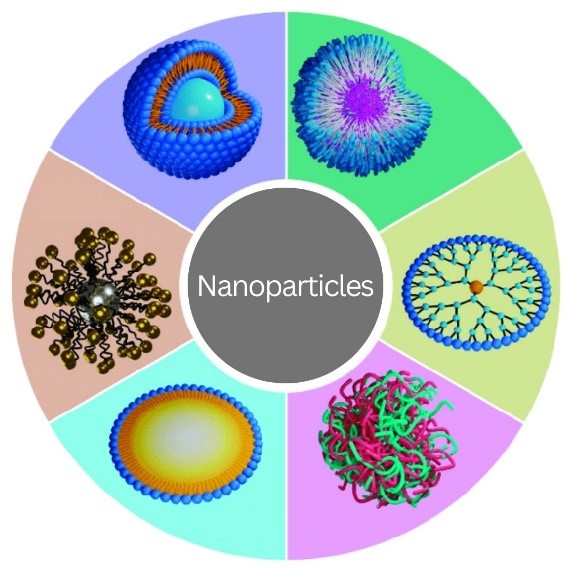







 Credit: Adapted from Compound Interest.
Credit: Adapted from Compound Interest.