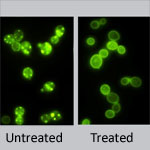
Scientists know that variations in certain genes can affect the way a person responds to medications. New research by Wolfgang Sadee ![]() at Ohio State University shows that drug responses also depend on previously overlooked parts of DNA—sections that regulate genes, but are not considered genes themselves. This study focused on an important enzyme abbreviated CYP2D6 that processes about one-fourth of all prescription drugs. Differences in the enzyme’s performance, which range from zilch to ultra-rapid, can dramatically alter the effectiveness and safety of certain medications. Researchers discovered two new genetic variants that impact CYP2D6 performance. One of these, located in a non-gene, regulatory region of DNA, doubles or even quadruples enzyme activity. Coupling these findings with genetic tests could help doctors better identify each patient’s CYP2D6 activity level, enabling more precise prescriptions. The findings also open up a whole new area of investigation into genetic factors that impact drug response.
at Ohio State University shows that drug responses also depend on previously overlooked parts of DNA—sections that regulate genes, but are not considered genes themselves. This study focused on an important enzyme abbreviated CYP2D6 that processes about one-fourth of all prescription drugs. Differences in the enzyme’s performance, which range from zilch to ultra-rapid, can dramatically alter the effectiveness and safety of certain medications. Researchers discovered two new genetic variants that impact CYP2D6 performance. One of these, located in a non-gene, regulatory region of DNA, doubles or even quadruples enzyme activity. Coupling these findings with genetic tests could help doctors better identify each patient’s CYP2D6 activity level, enabling more precise prescriptions. The findings also open up a whole new area of investigation into genetic factors that impact drug response.
This work also was funded by NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Learn more:
Ohio State University News Release (no longer available)