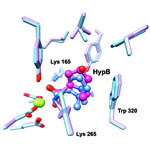
Sequencing the genomes of almost 7,000 organisms has identified more than 40 million proteins. But how do we figure out what all these proteins do? New results from an initiative led by John Gerlt of the University of Illinois suggest a possible method for identifying the functions of unknown enzymes, proteins that speed chemical reactions within cells. Using high-powered computing, the research team modeled how the structure of a mystery bacterial enzyme, HpbD, might fit like a puzzle piece into thousands of proteins in known metabolic pathways. Since an enzyme acts on other molecules, finding its target or substrate can shed light on its function. The new method narrowed HpbD’s candidate substrate down from more than 87,000 to only four. Follow-up lab work led to the actual substrate, tHypB, and determined the enzyme’s biological role. This combination of computational and experimental methods shows promise for uncovering the functions of many more proteins.
Learn more:
University of Illinois at Urbana-Champaign News Release
Gerlt Lab
Enzyme Function Initiative

