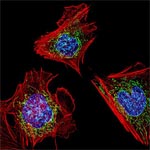
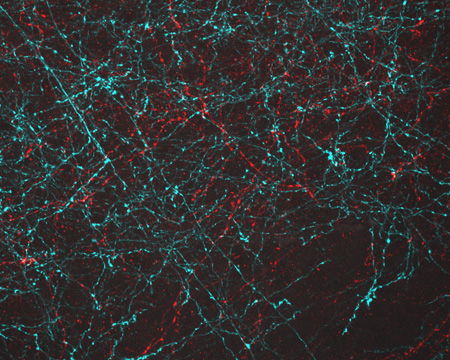
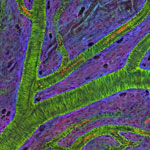
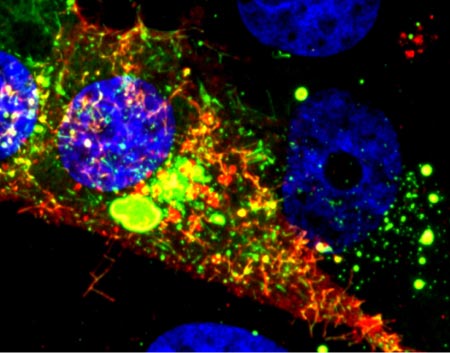
Even traveling cells need help with directions. In fact, it’s crucial. For processes such as wound healing and organ development to take place, cells must be able to efficiently move throughout organisms. Receptor proteins on a cell’s surface rely on navigational signals from molecules called netrins to point them in the right direction.
The receptors don’t just sit around waiting for a signal. Studying the simple worm C. elegans, David Sherwood ![]() and his research team at Duke University discovered that in the absence of netrin, the receptors rapidly cluster and reassemble in different areas of the cell’s membrane. When the receptors finally detect a netrin signal, they stabilize and correctly orient the cell. The finding might point to new ways to interfere with cells’ built-in navigation systems to hamper cell migration in metastatic cancer or encourage the regrowth of damaged cells in neurodegenerative diseases such as Parkinson’s.
and his research team at Duke University discovered that in the absence of netrin, the receptors rapidly cluster and reassemble in different areas of the cell’s membrane. When the receptors finally detect a netrin signal, they stabilize and correctly orient the cell. The finding might point to new ways to interfere with cells’ built-in navigation systems to hamper cell migration in metastatic cancer or encourage the regrowth of damaged cells in neurodegenerative diseases such as Parkinson’s.
This research was funded in part by NIH under grants R01GM100083 and P40OD010440.