Like a successful business networker, a cell’s endoplasmic reticulum (ER) is the structure that reaches out—quite literally—to form connections with many different parts of a cell. In several important ways, the ER enables those other parts, or organelles, to do their jobs. Exciting new images of this key member of the cellular workforce may clarify how it performs its roles. Such knowledge will also help studies of motor neuron and other disorders, such as amyotrophic lateral sclerosis (ALS), that are associated with abnormalities in ER functioning.
Structure Follows Function
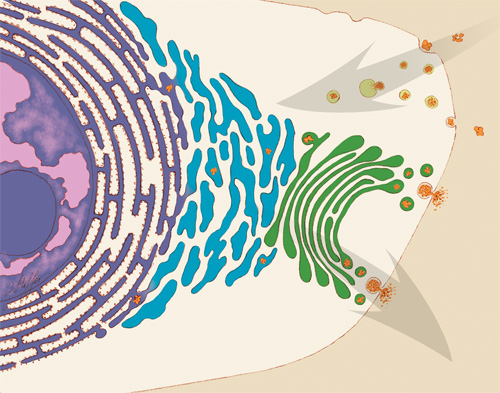
An illustration of some of the jobs that the endoplasmic reticulum (ER) performs in the cell. Some ER membranes (purple) host ribosomes on their surface. Other ER membranes (blue) extending into the cytoplasm are the site of lipid synthesis and protein folding. The ER passes on newly created lipids and proteins to the Golgi apparatus (green), which packages them into vesicles for distribution throughout the cell. Credit: Judith Stoffer.
Initiated in 1965, the Postdoctoral Research Associate Program (PRAT) is a competitive postdoctoral fellowship program to pursue research in one of the laboratories of the National Institutes of Health. PRAT is a 3-year program providing outstanding laboratory experiences, access to NIH’s extensive resources, mentorship, career development activities and networking. The program places special emphasis on training fellows in all areas supported by NIGMS, including cell biology, biophysics, genetics, developmental biology, pharmacology, physiology, biological chemistry, computational biology, immunology, neuroscience, technology development and bioinformatics
The ER is a continuous membrane that extends like a net from the envelope of the nucleus outward to the cell membrane. Tiny RNA- and protein-laden particles called ribosomes sit on its surface in some places, translating genetic code from the nucleus into amino acid chains. The chains then get folded inside the ER into their three-dimensional protein structures and delivered to the ER membrane or to other organelles to start their work. The ER is also the site where lipids—essential elements of the membranes within and surrounding a cell—are made. The ER interacts with the cytoskeleton—a network of protein fibers that gives the cell its shape—when a cell divides, moves or changes shape. Further, the ER stores calcium ions in cells, which are vital for signaling and other work.
To do so many jobs, the ER needs a flexible structure that can adapt quickly in response to changing situations. It also needs a lot of surface area where lipids and proteins can be made and stored. Scientists have thought that ER structure combined nets of tubules, or small tubes, with areas of membrane sheets. However, recent NIGMS PRAT (Postdoctoral Research Associate; see side bar) fellow Aubrey Weigel, working with her mentor and former PRAT fellow Jennifer Lippincott-Schwartz of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (currently at the Howard Hughes Medical Institute in Virginia) and colleagues, including Nobel laureate Eric Betzig, wondered whether limitations in existing imaging technologies were hiding a better answer to how the ER meets its surface-area structural needs in the periphery, the portion of the cell not immediately surrounding the nucleus. Continue reading “The Endoplasmic Reticulum: Networking Inside the Cell”
 Firefly. Credit: Stock photo.
Firefly. Credit: Stock photo.