Cells are the ultimate smart material. They can sense the demands being placed on them during critical life processes and then respond by strengthening, remodeling or self-repairing, for instance. To do this, cells use “mechanosensory” systems similar to the cruise control that lets a car’s engine adjust its power output when going up or down hills.
Researchers are uncovering new details on cells’ molecular cruise control systems. By learning more about the inner workings of these systems, scientists hope ultimately to devise ways to tinker with them for therapeutic purposes.
Cell Fusion
To examine how cells fine-tune their architecture and force output during the merging or fusion of cells, Elizabeth Chen and Douglas Robinson of Johns Hopkins University teamed up with Daniel Fletcher of the University of California, Berkeley. Cell fusion is critical to many developmental and physiological processes, including fertilization, placenta formation, immune response, and skeletal muscle development and regeneration.
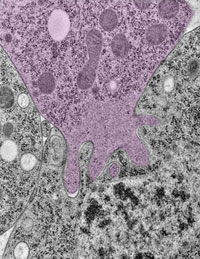
Using the fruit fly Drosophila melanogaster as a model system, Chen’s research group ![]() previously found that when two muscle cells merge during muscle development, fingerlike protrusions of one cell invade the territory of the other cell to promote fusion. In the new study, led by Chen, the researchers showed that cell fusion depends on the ability of the “receiving” cell to put up resistance against the invading cells.
previously found that when two muscle cells merge during muscle development, fingerlike protrusions of one cell invade the territory of the other cell to promote fusion. In the new study, led by Chen, the researchers showed that cell fusion depends on the ability of the “receiving” cell to put up resistance against the invading cells.
In fusing fruit fly cells, the scientists saw that in areas where the invading cells drilled in, the receiving cells quickly stiffened their cell skeletons, effectively pushing back. This mechanosensory response allows the outer membranes of the two cells to be pushed together and later fuse, Chen explains.
The team then explored the mechanisms underlying the stiffening response. They found that a protein called myosin II, which is part of the cell skeleton, senses the pushing force from the invading cell. Myosin II swarms to the fusion site and binds with fibers just beneath the cell membrane to put up the necessary resistance.
“We hope that by understanding the fusion process we can improve therapeutic strategies for degenerative muscle diseases,” Chen says. During normal development, hundreds or thousands of individual muscle cells must merge to form a skeletal muscle fiber. Figuring out how to promote merging could facilitate the development of therapies to regenerate injured or diseased skeletal muscle.
Shape Change
Robinson’s research group uses the amoeba Dictyostelium discoideum to investigate the fundamental principles of how cells change shape and how chemical and mechanical signals guide those shape changes. “Much of our work focuses on cytokinesis, the physical process in which one cell splits into two, as a way to shed light on how cells change shape in general,” Robinson says.
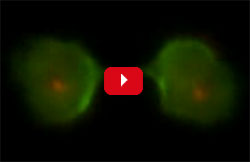
His team devised a way to use Dictyostelium cells to screen thousands of molecules for those that could tweak cell shape during cytokinesis. Such molecules, Robinson reasoned, could be useful in treating conditions as diverse as cancer and chronic obstructive pulmonary disease, which are marked by changes in cells’ mechanical properties.
The researchers identified one molecule, 4-HAP, that changed the location of myosin II in the cell skeleton. They then tested 4-HAP on lab-grown pancreatic cancer cells from patients with advanced disease, which their studies showed were softer than normal pancreas cells. The scientists found that the molecule affected myosin II in a way that made the cancer cells stiffer and decreased cell invasion and migration. By making roaming cancer cells firmer, a drug like 4-HAP might prevent malignant tumor cells from shape-shifting and squeezing through tissues to spread to new areas.
Both Robinson and Chen are working to gain more insights on how cells navigate the ups and downs of their daily lives. Only the future will show for sure what interesting and productive new roads their research may take.

