 Michael Boyce, associate professor of biochemistry at Duke University in Durham, North Carolina. Credit: Michael Boyce.
Michael Boyce, associate professor of biochemistry at Duke University in Durham, North Carolina. Credit: Michael Boyce.
Sugars aren’t merely energy sources for our cells. They also play important signaling roles through a process called glycosylation, where they attach to proteins and lipids as tags. Although these sugar tags, called glycans, impact many cellular processes, they have long been understudied due to technical challenges. Now, advances in analytical tools like mass spectrometry are enabling scientists to examine the enormous complexity of glycans. Other advances also allow researchers to synthesize complex sugars, providing them with standards for analytical experiments.
Dr. Michael Boyce, an associate professor of biochemistry at Duke University in Durham, North Carolina, and a recipient of the Presidential Early Career Award for Scientists and Engineers (PECASE), has begun using these new tools to explore the fascinating role of sugars in signaling.
“The attachment of sugars or sugar chains is the most abundant modification of proteins and lipids in all of nature,” says Boyce. “It impacts basically all cell biological processes.” In fact, abnormal glycosylation can be observed in almost all human diseases, including cancer, neurodegenerative conditions, and diabetes. Boyce’s lab focuses on glycosylation’s signaling role in such processes as cytoskeleton dynamics, protein stability, and vesicle trafficking, hoping to shed light on both normal physiology and disease.
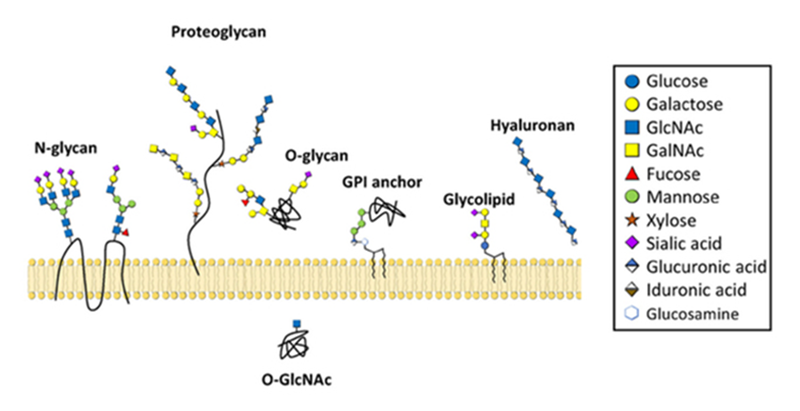 A cartoon depicting common types of glycans—structures composed of sugars—made by mammalian cells. Credit: Alex Broussard and Michael Boyce. CC BY-NC-SA-3.0.
A cartoon depicting common types of glycans—structures composed of sugars—made by mammalian cells. Credit: Alex Broussard and Michael Boyce. CC BY-NC-SA-3.0.
For example, one glycosylated protein is involved in degrading proteins that are part of the cytoskeleton. This protein is also mutated in the rare neurodegenerative disease giant axonal neuropathy. Because the protein is involved directly in the disease, understanding how it works—and how glycosylation affects its function—may inform the development of therapies.
For Boyce, the PECASE is meaningful because it recognizes not only his scientific contributions, but also his service to increase diversity, equity, and inclusion in the biomedical research workforce. Among other efforts at Duke, he has spearheaded a seminar series in his department that brings in biochemistry and cell biology faculty members from other institutions to talk about their science and efforts to promote diversity and inclusion.
Boyce is also co-chair of the American Society for Cell Biology’s Minorities Affairs Committee. The committee runs several federally funded programs for postdocs and junior faculty from underrepresented backgrounds that promote scientific and professional development, such as workshops for grant writing and job applications, and forums for networking and mentoring.
“I’m really glad that this kind of service is valued even at the highest levels of the government, because I think it’s an important thing for all scientists to contribute to inclusion, equity, and broad participation in the American research enterprise,” Boyce says.
Boyce’s research is supported in part by NIGMS grants R01GM117473 and R01GM118847, and NINDS grant R01NS111588.

