
“We scientists know very little of what can be known—I find that invigorating,” says Dylan Burnette, Ph.D., an associate professor of cell and developmental biology at Vanderbilt University School of Medicine in Nashville, Tennessee. “Most people find it exhausting, but I’m comfortable with not knowing all of biology’s secrets.” In an interview, Dr. Burnette shared his lab’s work on muscle cells, the knowledge he hopes readers take away from his research, and some advice to future scientists about being comfortable being wrong.
Q: How did you first become interested in science?
A: Unlike with other subjects (it took me a long time to learn how to read), I excelled at science. In third-grade science class, I knew every answer on the tests. It didn’t occur to me at the time, but I did well because I found it interesting. I decided I wanted to become a medical doctor that year. Back then, doctors were the only type of person who I thought did any type of science.
Q: What was your path to becoming a researcher?
A: After high school, I enrolled at the University of Georgia in Athens for an undergraduate degree in biology. There, I worked in the lab of Jacek Gaertig, Ph.D., studying the single-celled organism Tetrahymena thermophila, which uses cilia to move through its environment. This work made me excited to learn more about microtubules, which are found at the center of cilia and are part of the cytoskeleton that gives cells structural support.
My undergrad research experiences led me to pursue a Ph.D. from Yale University in New Haven, Connecticut. With my Ph.D. mentor, Paul Forscher, Ph.D., I studied neuronal growth cones. Nerve cells, also called neurons, send and receive information throughout the body in the form of electrical impulses. But to share information, neurons must make connections with other nearby neurons. They have long tail-like extensions called axons that grow off the cell body to send messages, and the tip of each axon has a growth cone that samples the local environment and guides growth to the nearest neuron to make connections. This process depends on the activity of microtubules and actin filaments, another part of the cytoskeleton.
After finishing at Yale, I went on to a postdoctoral position with Jennifer Lippincott-Schwartz, Ph.D., at the National Institute of Child Health and Human Development. There, I continued to study the cytoskeleton, which felt natural to me, as I had been studying it for more than a decade by then. My project aimed to understand how a cell moves and what forces propel it forward. The outward bulging of a cell membrane, one of the steps of movement, is heavily dependent on the growth and contraction of the cytoskeleton.
Q: What does your lab study?
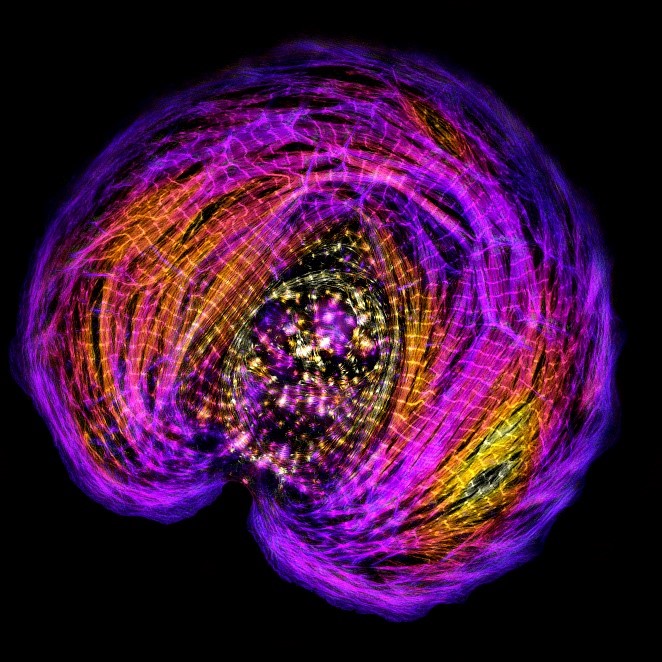
A: My lab studies muscle cells. I had intended to continue studying how non-muscle cells change their shape, but a loss in my family changed my research plans. When I was engaged to my now-wife, her brother died suddenly of a condition called hypertrophic cardiomyopathy. In this condition, the heart can’t pump blood effectively because the muscles of the heart wall have thickened. This thickening is often caused by inherited variants of genes that lead to an abnormal form of the protein myosin, which, alongside actin, is responsible for heart muscle contraction. The abnormal protein causes muscle cells to grow large and generate more force than necessary for contraction. Seeing my wife’s brother succumb to hypertrophic cardiomyopathy led me to change gears and study muscle cells.
Now, I’m researching the force-generating system in a muscle cell called the sarcomere. Sarcomeres are small units containing all the proteins and filaments needed for muscle contraction, including actin and myosin. These units assemble end to end into long, repeating strands, called myofibrils. When seen under a microscope, myofibrils are striated (meaning they alternate between light and dark regions), which correspond to the tightly controlled position of the actin and myosin filaments of each sarcomere.
Despite knowing the highly repeated and controlled structure of sarcomeres, researchers don’t fully understand how sarcomeres assemble. To study this, my lab members and I worked with immature human heart muscle cells grown in cell culture. We watched these cells assemble their sarcomeres in real time using high-resolution microscopy. Since then, we’ve studied sarcomere assembly in zebrafish and found that they use the same mechanism as humans.
Q: If you could choose one takeaway message for someone to learn as they read about your research, what would it be?
A: It would be that basic biomedical research such as mine focuses on the unanswered questions in biology, which lead to the generation of new, never-before-asked questions, which lead to new ways of thinking and new discoveries that may then lead to disease treatment. I’m not directly trying to cure disease at this point in my career. Instead, I’m trying to understand how a muscle cell gets bigger or smaller or changes its shape, and what’s needed for those changes to happen.
Q: Why is microscopy an important complement to your research?
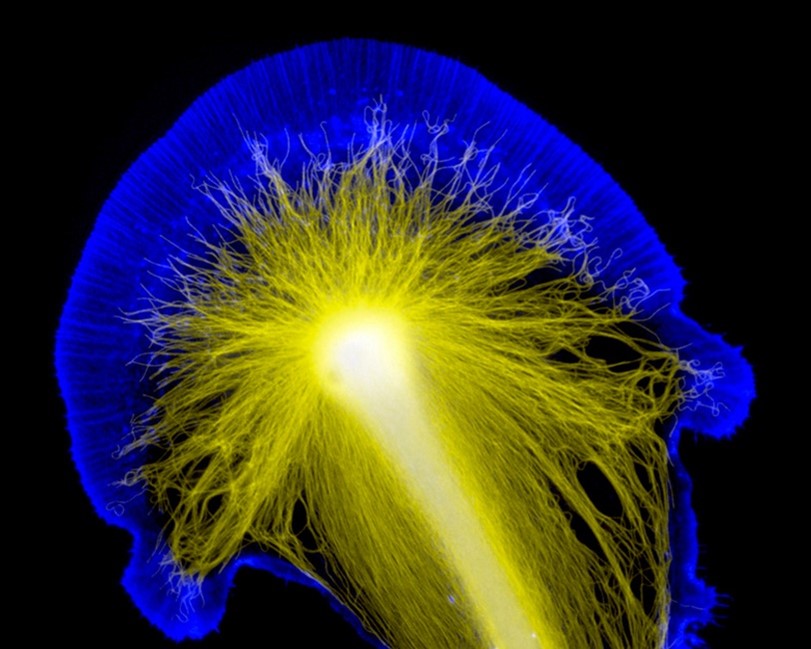
A: The first really beautiful image I ever took in the lab was of a neuronal growth cone during my Ph.D. I remember at the time trying to explain to my mother what I was researching. My explanation was pretty chaotic and unhelpful for her, until I showed her that image. With it, it was easier to explain what an axon or a cell body was, and where they are in relation to each other.
Ever since then, I’ve reminded myself how much easier it is to explain difficult concepts when you have a prop. Even now, in my presentations or publications, I’ll show example images alongside graphs. When people say a picture is worth a thousand words, they’re wrong. It’s worth even more than that. Plus, pictures are fun to look at.
Q: What advice would you give students who are interested in pursuing a career in science?
A: Find a mentor who fosters an environment where it’s okay to be wrong. Scientists’ hypotheses turn out to be incorrect all the time. But in the process of testing those hypotheses, we generate all of this worthwhile data that can be used to formulate new ones. That process of being wrong but having a chance to figure out what the right question is—that’s what I live for. I would tell students to find a mentor who also embraces that side of the scientific process.
Dr. Burnette’s work is supported by the NIGMS Maximizing Investigators’ Research Award program through grant R35GM125028.





Does fisetin improve the neural growth cone?
Hi Virginia, thanks for your question. Fisetin is being studied for its neuroprotective effects, so while researchers may identify a link between it and neuronal growth cones, it doesn’t seem that thay’ve identified one yet. This would be a great question for Dr. Burnette!