Numbers are everywhere in chemistry. You can’t balance equations, determine limiting reactants, or calculate percent yields without them. So, let’s dive into some of the significant figures in chemistry!
3
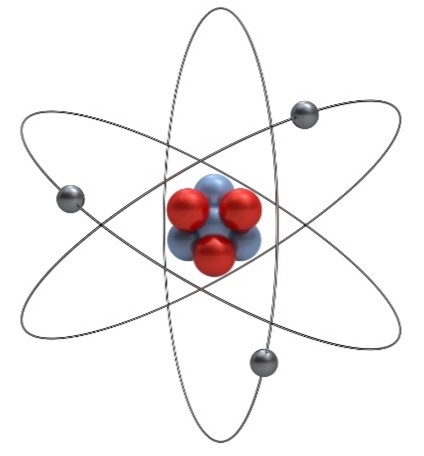
That’s the number of different types of particles—protons, neutrons, and electrons—that make up atoms, the basic unit of all matter. Protons are positively charged, neutrons are neutral, and electrons are negatively charged. The number of protons in an atom determines what element it is, and atoms usually have an equal number of protons and electrons. Atoms can have different numbers of neutrons, though, and atoms with the same number of protons and different numbers of neutrons are called isotopes. Protons and neutrons make up the core—or nucleus—of an atom, and electrons orbit around them.
4.9 Million
That’s how many miles per hour the electron in one hydrogen atom in a molecule of water is moving. At that rate, the electron could make it from New York City to Los Angeles in about 2 seconds!
118
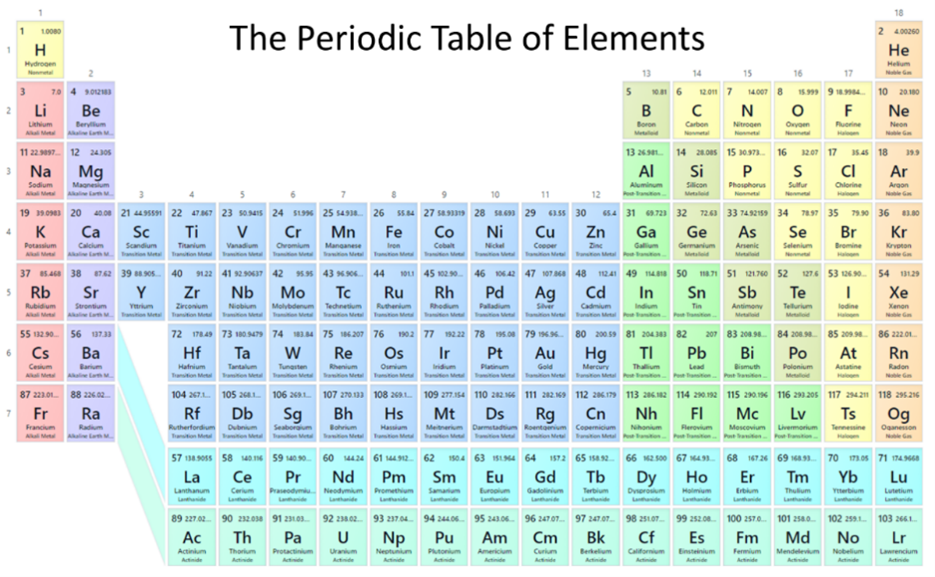
That’s the number of elements on the periodic table—the system that Dmitri Mendeleev created to organize all known (and even unknown) elements by their properties. Elements are arranged in rows based on the number of protons in their nucleus, and the different columns tell us about an element’s reactivity. For instance, elements in the far-right column are the noble gases—they’re naturally unionized, unreactive gases because they have a full set of electrons around their nucleus. Next to the noble gases are halogens, which have room for one more electron around their nucleus. Halogens tend to take on that extra electron to become negatively charged. On the opposite end of the table are the alkali metals, with one electron around their nucleus to give up. You may be familiar with elements from both of these groups because they’re what make up table salt: sodium chloride. As an alkali metal, sodium is positively charged, and, as a halogen, chlorine (called chloride when ionized) is negatively charged. Together, they form a strong ionic bond to become the seasoning you use on your food.
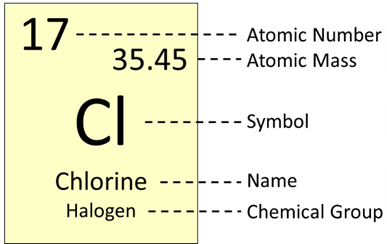
The periodic table provides detailed informtion on each element. Using chlorine as an example, it’s atomic number is 17, which means it has 17 protons in its nucleus. And because elements are ordered based on their number of protons, it’s also seventeenth on the table as you go across the rows. Chlorine’s atomic mass is 35.45, which is useful to chemists as they calculate the masses of molecules that have chlorine atoms in them or as they determine how much of a chemical to put into their reactions. Each element has its own one- or two-letter symbol, which you will encounter as you write chemical formulas or draw molecular structures. Most symbols have a clear reference to the element’s name, but others are derived from the name of the element in a different language—like “Pb” is the symbol for lead, based on its Latin name plumbum. Some periodic tables add additional details into their element boxes or color code elements based on certain characteristics.
2
That’s the number of elements that exist naturally as a liquid—just mercury and bromine. Eleven others exist as a gas (the noble gases, plus hydrogen, nitrogen, oxygen, fluorine, and chlorine). And the rest are solids.
4
That’s how many different types of chemical bonds there are: covalent, ionic, metallic, and hydrogen. When two atoms share electrons, they form covalent bonds. For example, one oxygen atom forms two covalent bonds with two hydrogen atoms to make H2O—which you probably know as water. The names of the other three bonds give away what types of atoms they involve: Ionic bonds are between two oppositely charged ions; metal atoms form metallic bonds; and hydrogen bonds are strong attractions from a hydrogen atom to another atom, which also happen in water, though hydrogen bonds are much weaker than covalent bonds.
12
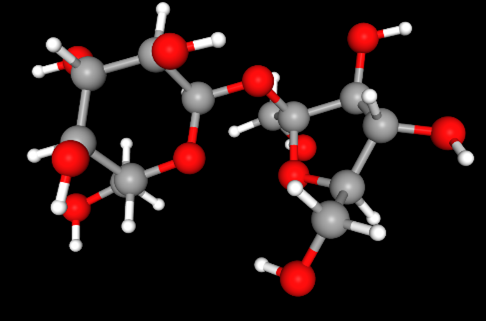
That’s the number of carbon atoms in a molecule of sucrose, or table sugar. Its chemical formula tells us this information with the subscript 12: C12H22O11. So, a molecule of sucrose also includes 22 hydrogen and 11 oxygen atoms. But don’t confuse subscripts with superscripts that tell charge, like oxygen’s negative two charge: O-2.
6.02 x 1023
That’s Avogadro’s number. It’s the number of elementary entities, such as atoms or molecules, in the unit of measurement called a mole. Chemists use moles to calculate the amount of material they need in a reaction. An extra unit of measure may not sound necessary, but because reactions often require specific ratios of different components and because different elements and molecules have different masses, standardizing everything to a mole helps scientists ensure they’re including the accurate amounts of reagents.
34
That’s the number of new small molecule medicines that the Food and Drug Administration (FDA) approved in 2023, along with 17 proteins and 4 oligonucleotides. Most medicines on the market have historically been small molecules—chemical compounds with low molecular weights. And while large, biologic medicines, like antibodies, vaccines, proteins, or nucleic acids, have recently become more common, they’re often not able to be taken by mouth or get into cells like small molecules can. FDA approval is the last step in the drug development process for new medications to become available to patients—but the FDA still monitors them once they’re on the market to confirm their safety. Basic science, like the research that NIGMS supports, usually occurs in the very early steps of this process but is often critical to the discovery of new targets or new methods to make these new molecules.

116
That’s the total number of Nobel Prizes that have been awarded in chemistry to a total of 197 laureates, 51 of whom NIGMS funded. They’ve ranged from the ages of 35 to 97 at the time of their awards.
If you liked this post, check out our other “By the Numbers” posts!



 Credit:
Credit: