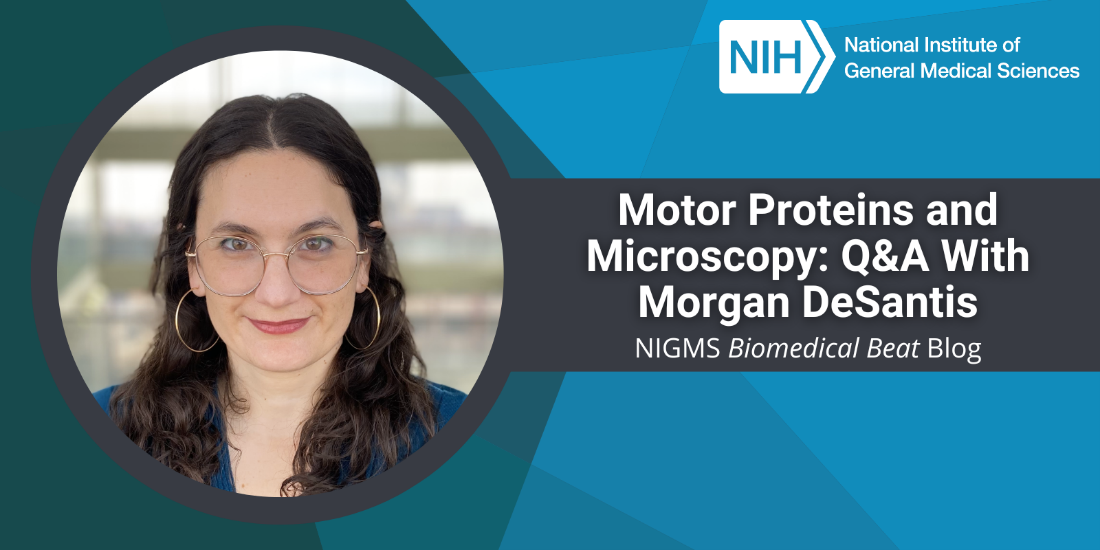
“I remember thinking in my first cellular biology class how impossibly beautiful it is that there are tiny machines in our bodies doing work,” says Morgan DeSantis, Ph.D., an assistant professor of molecular, cellular, and developmental biology at the University of Michigan in Ann Arbor. We talked with Dr. DeSantis about how her career in science almost didn’t happen, how happy she is that it did, and what she’s learning through her research on molecular machines.
Q: How did you become interested in science?
A: I wasn’t remotely interested in science in high school—I was a self-identified artist. I went to the College of Wooster in Ohio with the sole purpose of studying art and doing pottery. But one day during my freshman year, a box with all the pieces I made throughout the year fell, and everything inside broke. It’s hard to describe the emotions I felt that day, but something changed in me, and I realized pottery wasn’t for me. I couldn’t start the projects over, and I didn’t want to drop out and move back home. So, I decided to become a medical doctor.
Continue reading “Motor Proteins and Microscopy: Q&A With Morgan DeSantis”