It’s National Chemistry Week! To celebrate, we’re looking back at a few recent blog posts highlighting elements important for human health and scientific research. Check out the posts and tell us what your favorite element is in the comments section!
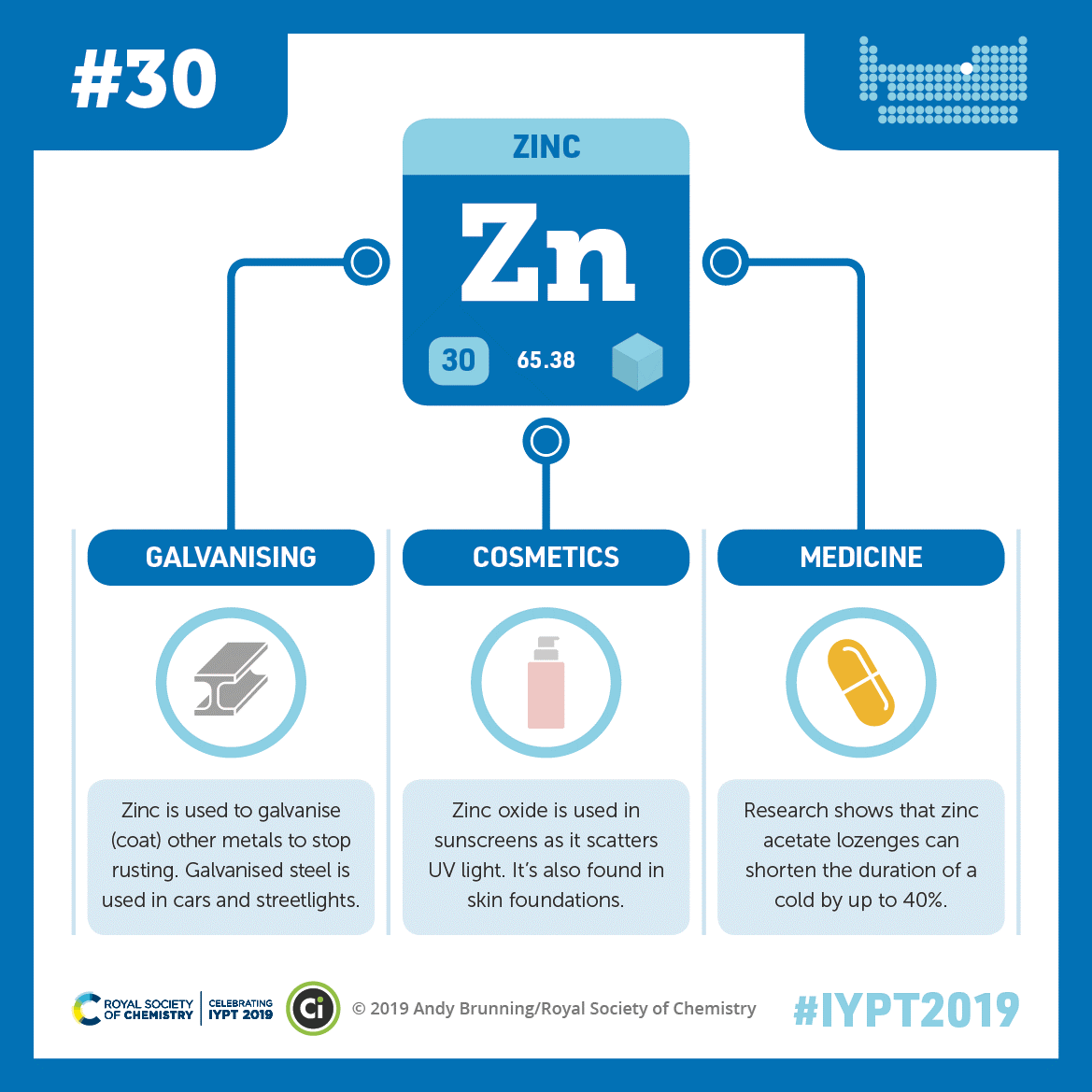
 Credit: Adapted from Compound Interest. CC BY-NC-ND 4.0.
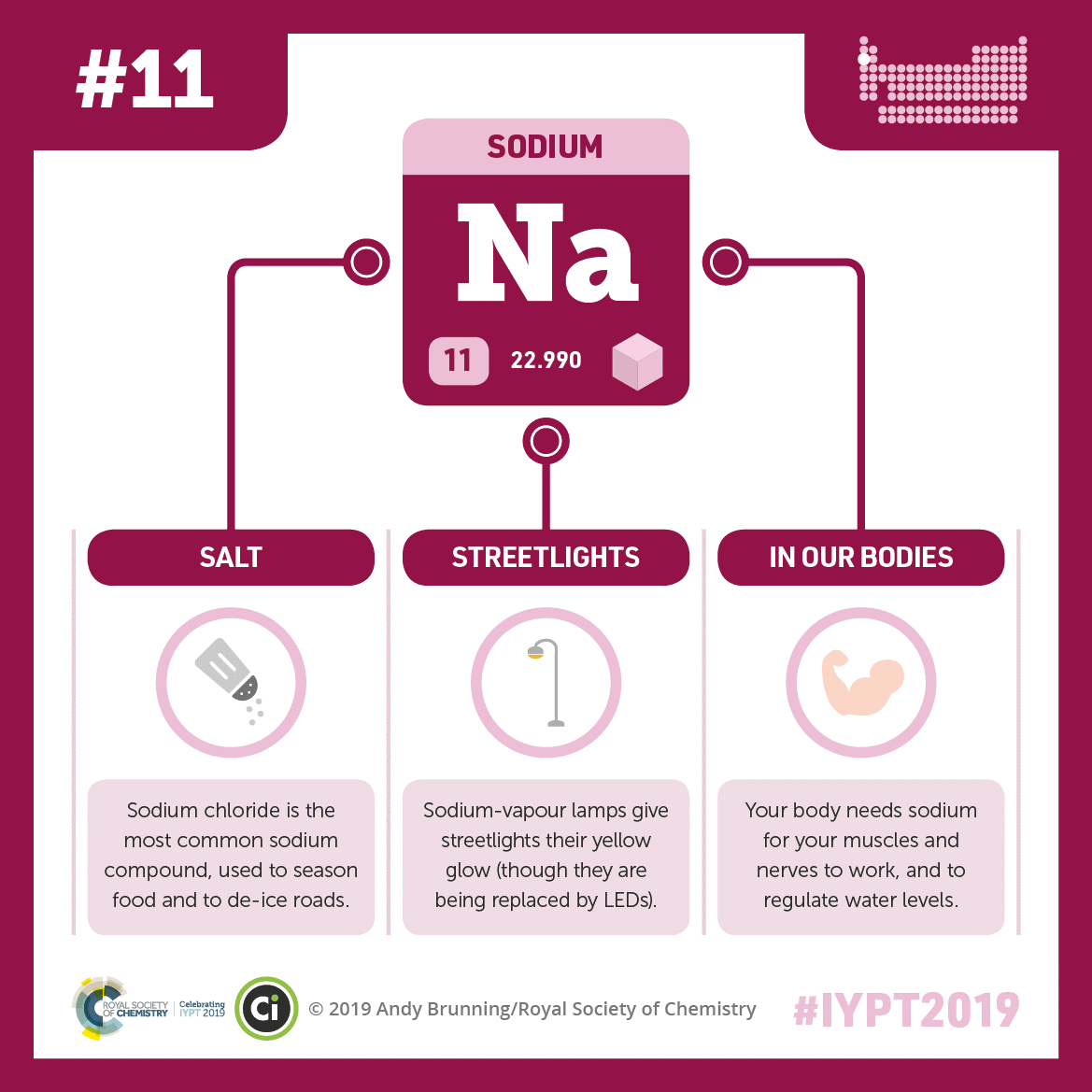
Credit: Adapted from Compound Interest. CC BY-NC-ND 4.0.
Got Calcium?
Calcium is the most abundant mineral in our bodies. It’s essential for lots of important functions—including keeping bones strong and allowing muscles to move. Even clicking on this post to learn more about its many roles requires calcium!
Continue reading “A Periodic Look at Elements”











 Credit: Adapted from Compound Interest.
Credit: Adapted from Compound Interest.