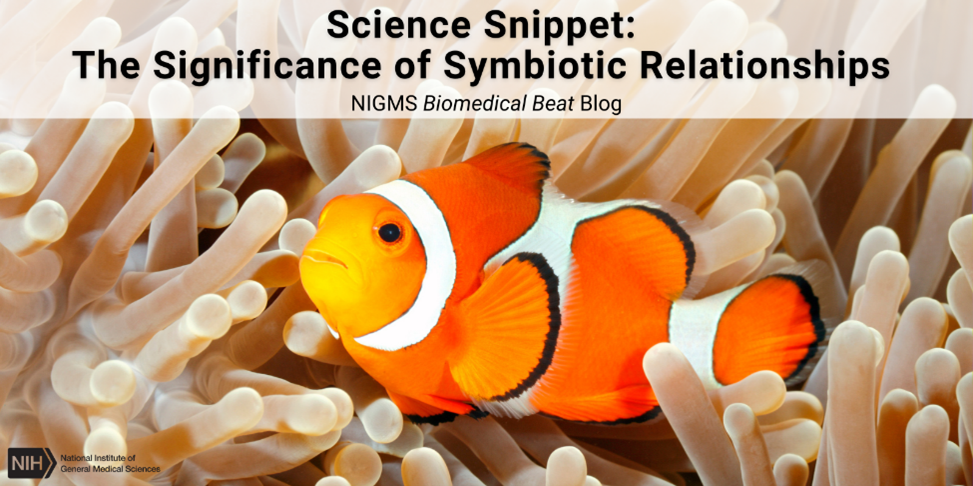
Relationships are complicated, even in nature. Two unrelated species living close together and interacting for survival is called symbiosis. There are three types of symbiotic relationships: mutualism, commensalism, and parasitism.

In a mutualistic relationship, both organisms benefit from the interaction. One example is the relationship between honeybees and flowers. Honeybees drink nectar from flowers, collecting and carrying pollen as they fly from one flower to another. Nectar allows bees to make honey, and spreading pollen helps flowers reproduce. Another example of a mutualistic relationship is between clownfish and sea anemones. The sea anemone provides protection and shelter, while clownfish waste provides the sea anemone with nutrients.
Continue reading “Science Snippet: The Significance of Symbiotic Relationships”