Every day, our cells must produce all the various molecules they need to stay alive. But the chemical reactions to create these molecules can’t occur without help—which is where enzymes come in. Enzymes are biological catalysts, meaning they speed up the rate of specific chemical reactions by reducing the amount of energy needed for the reaction to occur. Most enzymes are proteins, but some RNA molecules can also act as enzymes.
Thousands of different enzymes catalyze the vast range of reactions that take place within cells, but each enzyme typically supports one of the following types of tasks:
- Joining two molecules together by making a new chemical bond
- Breaking down a molecule into two or more new molecules by splitting a chemical bond
- Adding or removing specific groups of atoms, called functional groups, to or from a molecule
- Changing the overall shape of a molecule without adding or removing atoms
For example, kinases are enzymes that add phosphate groups (a phosphorous atom bonded to four oxygen atoms) to molecules. Conversely, phosphatases are a type of enzyme that removes phosphate groups from molecules. The addition and subtraction of phosphate groups can activate or deactivate a signaling molecule or even a protein. Cells’ major source of energy, adenosine triphosphate (ATP), is activated through the addition of the third phosphate group (hence, the triphosphate in ATP’s name) and deactivated when it’s removed. Similarly, depending on its current needs or to adapt to changes in its environment, a cell can activate or deactivate proteins—including enzymes—by adding or removing phosphate groups.
Some enzymes don’t function properly without the help of a second molecule called a cofactor. Examples of cofactors include metals, such as copper or manganese, and vitamins, such as vitamin B2. Our bodies can’t produce most of these molecules, so we must get them from our diet.
The Perfect Fit
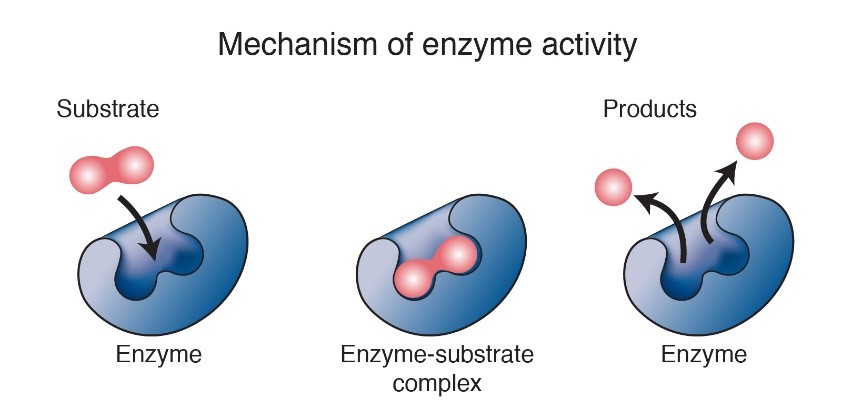
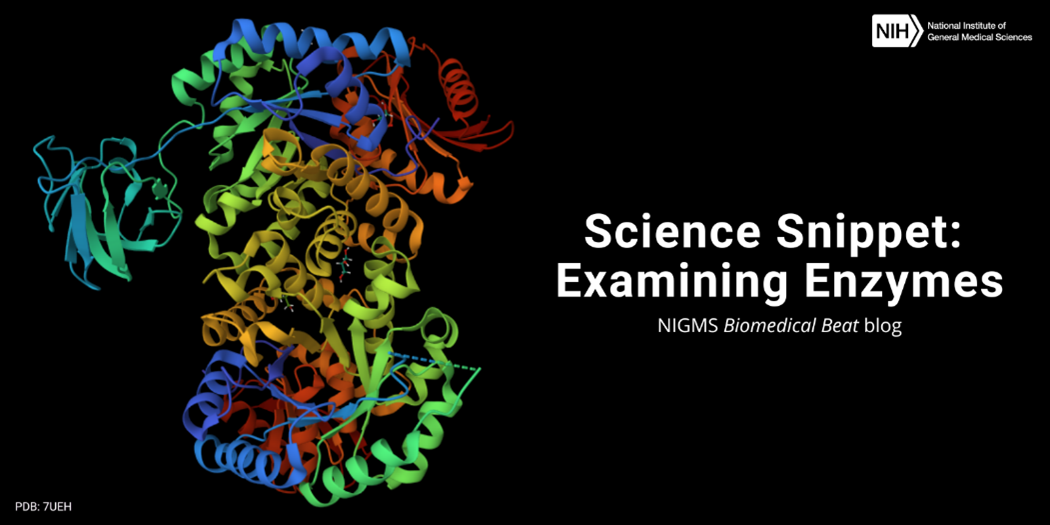
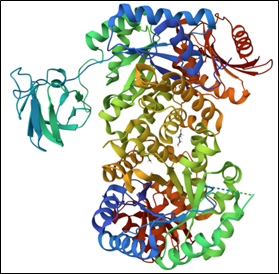
A groove on an enzyme’s surface, called an active site, is key to its functionality. This groove temporarily holds the substrate—the specific molecule the enzyme acts on. Only an enzyme’s substrate will fit perfectly within its active site.
When a substrate enters an active site, the substrate and enzyme interact in a way that changes the shape of the substrate slightly. When this happens, the energy needed to catalyze the reaction reduces, allowing chemical bonds to easily form or break. After, the final product(s) leave the enzyme, which is now free to bind to another substrate molecule.
Protein-based enzymes, like all proteins, are made up of chains of amino acids. There are 20 amino acids commonly found in nature, and each has a unique attachment called a side chain that dictates the amino acid’s behavior and affects an enzyme’s overall shape. If the wrong amino acid joins a growing chain, which can happen due to a variant in a person’s DNA or through mistakes in protein synthesis, the resulting enzyme may be misshapen and interact incorrectly with its substrate. Diseases can occur when enzymes don’t work normally. For example, Fabry disease is caused by ineffective versions of the enzyme alpha-galactosidase-A, which typically breaks down large lipids into smaller components. Without the effective version of this enzyme, lipids pile up in cells and may damage them.
NIGMS-Funded Research
NIGMS supports many scientists studying enzymes. Some of their research projects aim to:
- Learn how enzymes control DNA replication during cell division, which is important to prevent mistakes in DNA that may lead to cancer
- Identify enzymes found in beneficial gut bacteria that need metal ions—such as tungsten—to do their jobs, which could support a deeper understanding of the gut microbiome
- Further understand the interactions between a substrate and an active site, which may help other researchers design medicines that block or enhance an enzyme’s activity to treat disease
- Investigate bacterial proteins that block enzymes of the human immune system, which could enable the development of therapies to prevent or treat infections
Learn about other scientific terms with the NIGMS glossary.