“I think there’s a very creative side to science, in figuring out how to approach a problem, which I find really engaging,” says Mia Huang, Ph.D., an associate professor of chemistry at the Scripps Research Institute in La Jolla, California. In an interview, Dr. Huang discussed her shift in interest from medicine to science, her graduate school work on nature-inspired antifreeze molecules, and her lab’s exploration of the roles of sugar-coated proteins in our bodies.
Get to Know Dr. Huang
- Coffee or tea? Coffee
- Favorite music genre? EDM
- Cats or dogs? Dogs—I’m a proud mom to a 15-pound Bernedoodle
- Rainy or sunny? Sunny
- What was your childhood dream job? Scientist—I’m living the dream!
- Favorite hobby? Playing video games
- Favorite piece of lab safety equipment? Safety goggles
- A scientist (past or present) you’d like to meet? Gilbert Ashwell and Anatol Morell (accidentally co-discovered the asialoglycoprotein receptor)
Q: How did you first become interested in science?
A: I became interested in science as a way to get into medicine because I’d grown up thinking I wanted to be a medical doctor. When I was young, I also watched this cartoon called Dexter’s Laboratory, which was my first impression of what being a scientist might be like, and the show made me feel like that could be an interesting career path.
Q: What was your path to becoming a researcher?
A: I was a biochemistry major at the City University of New York, Queens College, which required a research laboratory experience as part of the curriculum. That was my first experience with the scientific lifestyle, and I thoroughly enjoyed the process of problem-solving and the potential for creating drugs and discovering how molecules act in the cell. As I was preparing for the Medical College Admission Test (MCAT), I realized that I might prefer being a scientist to being a physician.
I began exploring graduate school for chemistry, but at that point, I wasn’t very clear on the differences between a master’s degree and a Ph.D., apart from the years that were required. I was afraid of the 5-year time commitment for the Ph.D., and I recall asking one of the graduate students in the lab how she decided to commit 5 years to a Ph.D. She said, “What else are you going to do?” That jostled me a little bit, and I realized that although 5 years seemed like a long time, I couldn’t think of anything else I wanted to do career-wise beyond spending time in a lab and being engaged in the scientific process. So, I decided to apply to both master’s and Ph.D. programs. I landed at New York University as a master’s student and then slowly transitioned into the Ph.D. program.
Q: What did you study for your Ph.D. research?
A: I focused on designing molecules called peptoids to do certain jobs. Peptoids look similar to natural peptides, but they’re slightly chemically modified. Natural peptides are now more recognized as promising drug molecules, but when I was a student, the thinking was that they wouldn’t make good medicines because enzymes in the body could easily degrade them and make them nonfunctional. Peptoids’ chemical modifications make them resistant to these enzymes, and they’re easier than peptides to make in a lab.
I worked on developing peptoids into antimicrobial molecules. I also got involved in a fascinating project designing peptoids to be antifreeze molecules. Amphibians and fish that live in very cold environments naturally have antifreeze glycopeptides—peptides with sugar molecules attached to them. The glycan (sugar) groups on these glycopeptides interact with tiny particles of ice to inhibit their growth. I designed similar peptoids in the lab and used X-rays to look at how they could interact with ice particles.
Q: What were the next steps in your career?
A: I took a postdoctoral researcher position at Yale University in New Haven, Connecticut. My project there was designing synthetic molecules that could recruit components of the immune system, such as antibodies, to attack viruses or cancer cells. After my stint at Yale, I wanted to revisit my interest in glycopeptides that was sparked by my Ph.D. work with antifreeze glycopeptides, so I moved to a second postdoctoral position in a chemical glycobiology lab at the University of California, San Diego. In total, I did about 6 years of postdoctoral work, which not only addressed gaps in my training, but also solidified my interest in becoming a professor. I launched into the academic job market and ultimately accepted a position as an assistant professor at the Scripps Research Institute.
Q: What is your lab researching?
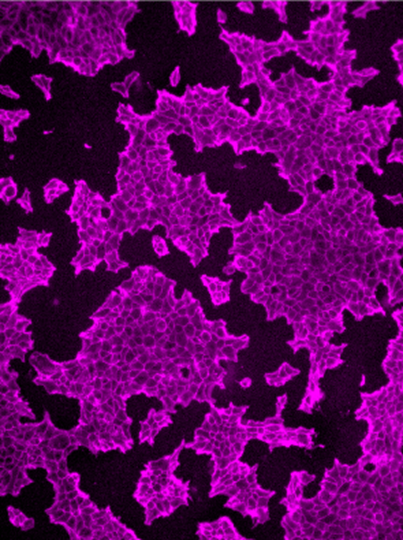
A: My lab is looking at how sugars regulate biology. One thing we focus on is capturing the binding complexes between glycoproteins and glycan-binding proteins on the surfaces of cells, which are quite difficult to isolate. We use chemistry-based techniques to capture the molecules so we can identify them using mass spectrometry. Once we’ve identified them, we can then use molecular biology techniques to either eliminate or increase the amount of glycoproteins in cells and see if they’re playing a role in a particular biological function.
In some cases, the interactions between certain glycan-binding proteins and glycoproteins may be involved in disease. My dream is that identifying functional complexes between glycan-binding proteins and glycoproteins enables us to find more precise ways to interrupt the interactions between these molecules, which could support the development of new medicines.
Q: What do you consider to be your biggest accomplishment so far?
A: With glycoproteins, it can be really difficult to understand which component of the molecule is responsible for its function. Is it the sugar portion? Is it the protein portion? Plus, these glycoproteins are normally attached to the surfaces of cells, but sometimes they’re freely floating around. Does the location of the molecule affect its function?
With the technology we’ve developed, we’ve been able to build molecules using specific proteins and sugars and choose whether or not the molecules are attached to the cell. This allows us to determine which factors are important for function. For example, we’ve learned that the kinds of sugars, type of protein, and whether the molecule is attached to the cell are all important for certain cancer cells to bind and spread in extracellular matrices.
Q: What kinds of challenges have you faced in your career?
A: As researchers, we’re trained well in how to approach scientific problems but not always people-related ones. You think you’re human enough to address these kinds of problems, but it’s different when you’re managing a lab and responsible for the team, keeping the whole enterprise running. I found that role to be quite challenging in the first few years of leading a lab. Having mentors at Scripps to ask for advice, participating in workshops provided by NIH, and using other resources really helped me grow as a manager.
Q: What advice would you give students who are interested in pursuing a career in science?
A: My greatest advice is to develop more of an imagination by reading and doing other activities to broaden your mind, like art. A lot of scientists approach the same problems with the same solutions, and sometimes what differentiates people is out-of-the-box thinking—creative ideas that are grounded in scientific knowledge but seem to come out of nowhere. A hypothesis starts by imagining things that may not yet be.
Dr. Huang’s research is supported by the NIGMS Maximizing Investigators’ Research Award program through grant R35GM142462. She also receives funding from the National Institute of Allergy and Infectious Diseases and the National Institute of Neurological Disorders and Stroke.