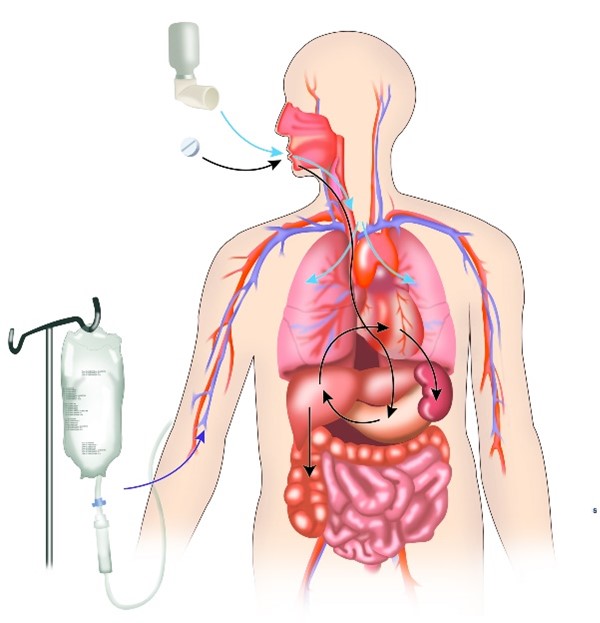
What we put into our bodies can affect how they function and what they do. For example, a sugary snack will probably make you feel differently than a high-protein meal. Similarly, different medicines elicit different responses in your body, and pharmacologists try to fine-tune each medicine to balance the desired (on-target) with the undesired (off-target) effects—a branch of pharmacology called pharmacodynamics.
Most medicines work by binding to a molecular target, usually proteins like receptors or enzymes, and either blocking or supporting its activity, which results in their therapeutic effects.
Continue reading “How Do Medicines Work?”