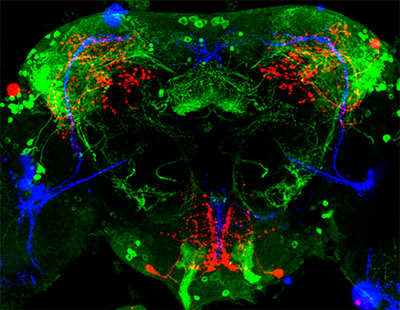
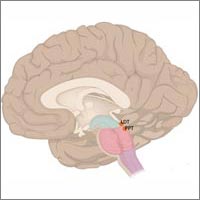
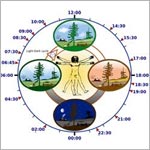
Genes and proteins run biological clocks that help keep daily rhythms in synch. Credit: Wikimedia Commons.
After you roll your clocks back by an hour this Sunday, you may feel tired. That’s because our bodies—more specifically, our circadian rhythms—need a little time to adjust. These daily cycles are run by a network of tiny, coordinated biological clocks.
NIGMS’ Mike Sesma tracks circadian rhythm research being conducted in labs across the country, and he shares a few timely details about our internal clocks:
1. They’re incredibly intricate.
Biological clocks are composed of genes and proteins that operate in a feedback loop. Clock genes contain instructions for making clock proteins, whose levels rise and fall in a regular cyclic pattern. This pattern in turn regulates the activity of the genes. Many of the results from circadian rhythm research this year have uncovered more parts of the molecular machinery that fine-tune the clock. Earlier in the month, we blogged about an RNA molecule that cues the internal clock.
2. Every organism has them—from algae to zebras.
Many of the clock genes and proteins are similar across species, allowing researchers to make important findings about human circadian processes by studying the clock components of organisms like fruit flies, bread mold and plants.
3. Whether we’re awake or asleep, our clocks keep ticking.
While they might get temporarily thrown off by changes in light or temperature, our clocks usually can reset themselves.
4. Nearly everything about how our body works is tied to biological clocks.
Our clocks influence alertness, hunger, metabolism, fertility, mood and other physiological conditions. For this reason, clock dysfunction is associated with various disorders, including insomnia, diabetes and depression. Even drug efficacy has been linked to our clocks: Studies have shown that some drugs might be more effective if given earlier in the day.
Learn more:
Circadian Rhythms Fact Sheet




 Cover of Pathways student magazine, third issue.
Cover of Pathways student magazine, third issue.
 A SEPA-funded resource about microbes. Credit: University of Nebraska, Lincoln.
A SEPA-funded resource about microbes. Credit: University of Nebraska, Lincoln.
 Cover of Pathways student magazine.
Cover of Pathways student magazine.