This time of year, many creepy crawlies take center stage to frighten people of all ages. To celebrate Halloween, we’ve conjured up a slideshow of fascinating but spooky species that NIGMS-funded scientists study. Some of these creatures drink blood like vampires, while others—frogs, worms, flies, and salamanders—are perfect cauldron ingredients for a witch’s brew. Check out the slideshow—if you dare!
Continue reading “Slideshow: Creepy Crawlies”Tag: Research Organisms
Trainee Colton Pelletier Builds a Rotifer-Studying Robot

During his time at Roger Williams University (RWU) in Bristol, Rhode Island, Colton Pelletier built a robot that will help simplify data collection for research projects in the lab he worked in—and others—for years to come. Aiding in Colton’s success in the lab was NIGMS funding through the Institutional Development Award (IDeA) Networks of Biomedical Research Excellence (INBRE) program. INBRE funds statewide networks of higher education in IDeA states such as Rhode Island, which have historically received low levels of NIH funding. The program supports faculty research, mentoring, student participation in research, and research infrastructure by connecting primarily undergraduate institutions with research-intensive universities in the state.
Continue reading “Trainee Colton Pelletier Builds a Rotifer-Studying Robot”Research Organism Superheroes: Hydras
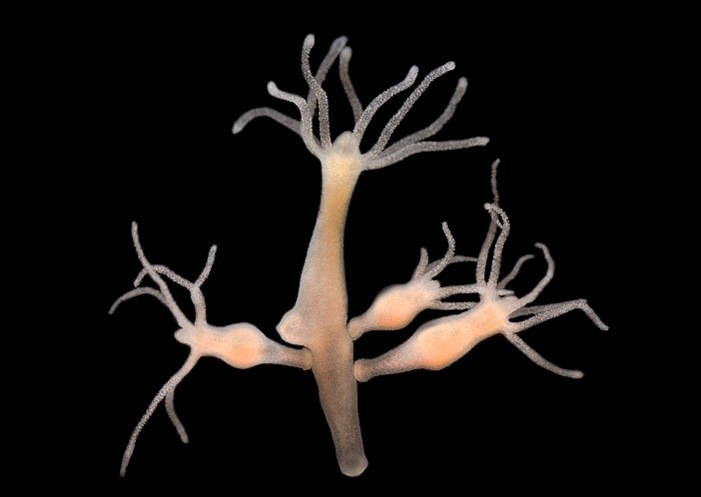
Hydras might look like they’re visitors from outer space, but they’re actually Earth-dwelling animals that can be found in fresh water, like ponds or gentle streams. The body of a hydra consists of a thin tubelike stalk that’s about an inch long with several tentacles extending from one end. Some hydras can grow an armlike extension that eventually pops off the main stem to become a new hydra.
Humans have studied hydras for hundreds of years. Antonie van Leeuwenhoek, one of the earliest known microscopists, first described them in 1703 when he looked at water samples under a microscope. You can see hydras—whose bodies are about the length of a paperclip—without them, but microscopes help researchers see their shape in better detail. Scientists commonly use hydras as research organisms because of their incredible ability to regrow lost body parts after injury through a process called regeneration.
Continue reading “Research Organism Superheroes: Hydras”Research Organism Superheroes: Hawaiian Bobtail Squid

The Hawaiian bobtail squid (Euprymna scolopes) is only about as big as a golf ball, but what it lacks in size, it makes up for in its superpower—an invisibility cloak to be exact. Thanks to its symbiotic relationship with the bioluminescent bacteria Vibrio fischeri, it’s able to seemingly disappear from its predators when swimming at night.
These super-squid live in the shallow coastal waters in the Pacific, like those around the Hawaiian Islands. They’re nocturnal, so they hunt their prey—small shrimp and other crustaceans—at night and hide, often by burying themselves in the sand, during the day while they rest. Although Hawaiian bobtail squid live their short 3-10 month lives around one another, they generally only interact for breeding, and even then, they only reproduce once in their lifetimes and die soon after reproduction.
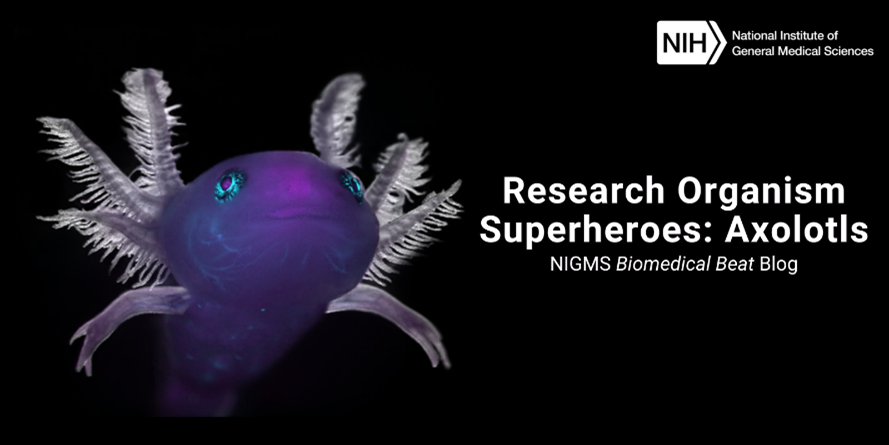
Continue reading “Research Organism Superheroes: Hawaiian Bobtail Squid”Research Organism Superheroes: Axolotls
The friendly-looking axolotl (Ambystoma mexicanum) doesn’t seem to have much in common with its namesake, Xolotl—the Aztec god of lightning, death, and fire. In fact, axolotls can regrow lost limbs and other body parts like organs and parts of their central nervous systems—which goes against the concept of death!
Continue reading “Research Organism Superheroes: Axolotls”Research Organism Superheroes: Fruit Flies

Those pesky little bugs flying around the overripe bananas in your kitchen may hold the key to understanding something new about how our bodies work. That’s right, the fruit fly (Drosophila melanogaster) is a widely used research organism in genetics because of its superpower of reproducing quickly with similar genes to people.
Researchers have been studying fruit flies for over a century for many reasons. First, they’re easy to please—just keep them at room temperature and feed them corn meal, sugar, and yeast (or those bananas on your counter!). Second, they reproduce more quickly and have shorter life cycles than larger organisms. A female can lay up to a hundred eggs in a day, and those eggs develop into mature adult flies within 10 to 12 days. A third reason is the simplicity of the fruit fly’s genome, which only has four pairs of chromosomes compared to the 23 in humans. And on a logistical note, the male and female flies are easy to tell apart (genetic studies often require separating males and females, which isn’t an easy feat in all organisms).
Continue reading “Research Organism Superheroes: Fruit Flies”Curiosity-Driven Science: Q&A With Saad Bhamla
What do worm blobs and insect pee have to do with human health? We talked to Saad Bhamla, Ph.D., assistant professor of chemical and biomolecular engineering at Georgia Institute of Technology (Georgia Tech) in Atlanta, to find out.
Q: What did your path to becoming a scientist look like?

A: I grew up in Dubai and did my undergraduate work in India, which is where I was first introduced to science. The science faculty members seemed to be having so much fun and would say things like “for the love of science,” but I couldn’t figure out what joy they were getting until I got a taste of it myself—then I was hooked. I like the idea that you can create a legacy doing science because someone can come along 100 years later and build on your work.
After undergrad, I went to Stanford University and earned my Ph.D. in the lab of Gerald Fuller, Ph.D., and then stayed at Stanford for postdoctoral work (postdoc) in the lab of Manu Prakash, Ph.D. In 2017, I joined the faculty at Georgia Tech. On paper, I’m a chemical engineer, but I describe myself as more of a biophysicist.
Continue reading “Curiosity-Driven Science: Q&A With Saad Bhamla”Research Organism Superheroes: Tardigrades

“Water bear” or “moss piglet”? No matter what you call them, tardigrades have secured the title of cutest invertebrate—at least in our book. They’re tiny creatures, averaging about the size of a grain of salt, so while you can spot them with the naked eye, using a microscope is the best way to see them. They earned their nickname of water bear and their official name (which comes from tardigradus, Latin for “slow walker”) because of the way they lumber slowly and deliberately on short, stubby legs.
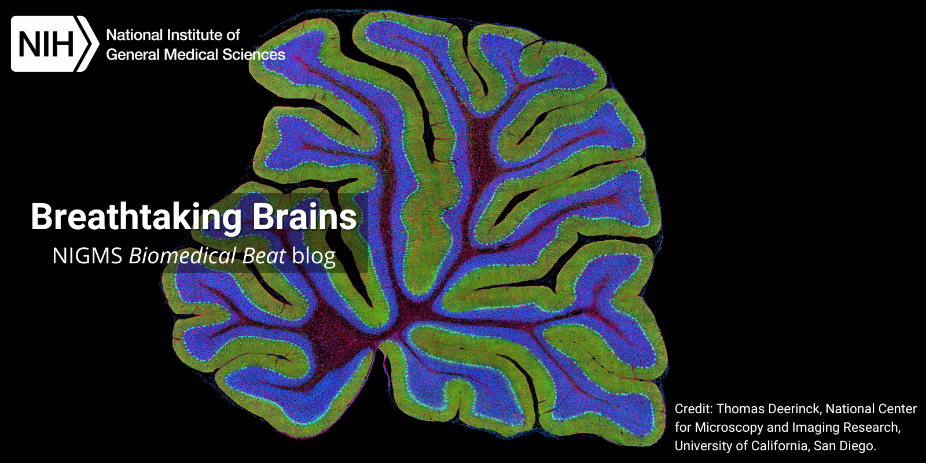
Continue reading “Research Organism Superheroes: Tardigrades”Slideshow: Breathtaking Brains
The average human brain is only about 3 pounds, but this complex organ punches well above its weight, acting as the control center for the whole body. Many of the brain’s intricacies still aren’t fully understood. To gain more insight into brain processes, scientists often peer into the brains of research organisms such as fruit flies and mice. These organisms have shed light on how our brains maintain circadian rhythms, how neuropsychiatric disorders develop, and more.
Continue reading “Slideshow: Breathtaking Brains”In Other Words: Media—Getting the News and Growing Cells
The word media may make many of us think about media outlets where we get our news or social media where we keep up with friends. But to biomedical researchers, media is a nutrient-rich liquid that fuels cell cultures—groups of cells grown in a lab. Scientists grow many types of cultures in media, from bacteria to human cells. They use these cultures to learn about basic biological processes and to develop and test new medicines.