Invasive fungal infections—the kind that infect the bloodstream, lung and brain—are inordinately deadly. A big part of the problem is the lack of drugs that are both effective against the fungi and nontoxic to humans.
The situation might change in the future though, thanks to the work of a multidisciplinary research team led by chemist Martin Burke at the University of Illinois. For years, the team has focused on an antifungal agent called amphotericin B (AmB for short). Although impressively lethal to fungi, AmB is also notoriously toxic to human cells.
Most recently, the research team chemically modified the drug to create compounds that kill fungi, but don’t disrupt human cells. The scientists explain it all in the latest issue of Nature Chemical Biology.
Invasive fungal infections are so intractable because most antifungal drugs aren’t completely effective. Plus, fungi have a tendency to develop resistance to them. AmB is a notable exception. Isolated 50 years ago from Venezuelan dirt, AmB has evaded resistance and remains highly effective. Unfortunately, it causes side effects so debilitating that some doctors call it “ampho-terrible.” At high doses, it is fatal.
For decades, scientists believed that AmB molecules kill fungal cells by forming membrane-piercing pores, or ion channels, through which the cells’ innards leak out. Last year, Burke’s group overturned this well-established concept using evidence from nuclear magnetic resonance, chemistry and cell-based experiments. The researchers showed that AmB molecules assemble outside cells into lattice-like structures. These structures act as powerful sponges, sucking vital lipid molecules, called ergosterol, right out of the fungal cell membrane, destroying the cell.
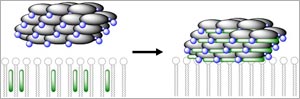
AmB’s power as an antifungal agent comes from its ability to bind strongly to ergosterol. Burke’s team found that AmB can also attach to the equivalent lipid in animals—cholesterol. The researchers suspect that AmB’s interactions with cholesterol are responsible for its noxious effects on humans.
In the newest paper, Burke and his colleagues used their detailed knowledge of AmB (and a chemical technique they developed) to create derivatives of the drug that bind strongly to fungal ergosterol, but not to human cholesterol. After extensive testing on two human cell types and in mice, the team zeroed in on two derivatives that have the long-sought combination of properties, namely efficacy (they are potent antifungals), selectivity (they destroy fungal cells but are nontoxic to host cells even at dosages that, for AmB, would be lethal) and the ability to thwart resistance.
If the AmB derivatives work equally well in humans, they could become a public health boon, especially to people with suppressed immune systems, who are especially vulnerable to invasive fungal infections. On a practical level, the compounds are easy to make from AmB, which is commercially available in large quantities.
More broadly, the work highlights a promising target for antimicrobial drugs—lipids. Currently, most drugs target protein molecules, which can readily change, potentially leading to drug resistance. According to this new research, lipids are not as malleable, so they might underlie a strategy for producing antimicrobial drugs that are less susceptible to drug resistance.
NIGMS’ director, Jon Lorsch, who has been following the team’s progress, calls the new work “a great demonstration of how a fundamental understanding of chemical and biochemical processes leads to important new opportunities to advance the treatment of deadly diseases.”

