When someone mentions aging, you may think of visible changes, like graying hair. Scientists can see signs of aging in cells, too. Understanding how basic cell processes are involved in aging is a first step to help people lead longer, healthier lives. NIGMS-funded researchers are discovering how aging cells change and applying this knowledge to health care.
Discovering the Wisdom of Worms
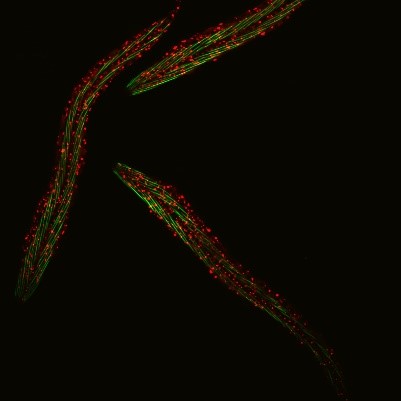 C. elegans with a ribosomal protein glowing red and muscle fibers glowing green. Credit: Hannah Somers, Mount Desert Island Biological Laboratory.
C. elegans with a ribosomal protein glowing red and muscle fibers glowing green. Credit: Hannah Somers, Mount Desert Island Biological Laboratory.
Aric Rogers, Ph.D., and Jarod Rollins, Ph.D., assistant professors of regenerative biology and medicine at Mount Desert Island (MDI) Biological Laboratory in Bar Harbor, Maine, are investigating aging by studying a tiny roundworm, Caenorhabditis elegans. Researchers often study C. elegans because, though it may seem drastically different from humans, it shares many genes and molecular pathways with us. Plus, its 2- to 3-week lifespan enables researchers to quickly see the effects of genetic or environmental factors on aging.
Drs. Rogers and Rollins investigate how C. elegans expresses genes differently under dietary restriction, enabling it to live longer. Understanding how genes are expressed when organisms live an extended life sheds light on the genetics underlying aging. This information could help researchers develop drugs or behavior modification programs that prolong life and delay the onset of age-related diseases such as heart disease, diabetes, cancer, and dementia.
In collaboration with colleagues at the MDI lab and elsewhere, Drs. Rogers and Rollins recently showed that C. elegans could live five times its normal life expectancy if the researchers altered genes in two molecular pathways that are affected by dietary restriction. The first is the insulin-like signaling pathway, which regulates sugar levels. The second is the target of rapamycin (TOR) pathway, which is involved in metabolism and other cell processes.
The researchers already knew that editing only the insulin-like signaling pathway doubles the worm’s life expectancy. And altering only the TOR pathway increases its life expectancy by about a third. But combining them had a much larger effect than they anticipated. This result suggests that targeting multiple pathways at the same time—similar to the way doctors often treat cancer and HIV with combination therapies—could be a powerful strategy to combat aging.
Helping Older Adults Stay Strong
Maintaining muscle is important for strength and mobility in older adults, and muscle cells also burn more calories than fat cells. But muscle protein synthesis slows over time, while the rate at which unexercised muscle degrades stays about the same. This means that older adults lose muscle more easily than younger adults.
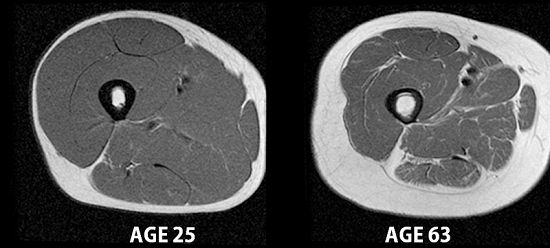 MRI images of a 25-year-old man and a 63-year-old man’s upper thighs, showing skeletal muscle (dark gray), fat, and bone. Credit: Carl Murphy and Sheri Coker, University of Alaska Fairbanks.
MRI images of a 25-year-old man and a 63-year-old man’s upper thighs, showing skeletal muscle (dark gray), fat, and bone. Credit: Carl Murphy and Sheri Coker, University of Alaska Fairbanks.
To prevent muscle loss, older adults need to consume enough essential amino acids, components of proteins that help build muscle. However, doctors often recommend weight loss through diet to those who are overweight or obese to help reduce health risks. And getting enough essential amino acids through a reduced-calorie diet can be difficult and can result in muscle loss.
To help patients retain muscle while dieting, Robert Coker, Ph.D., a professor of biology at the University of Alaska Fairbanks Institute of Arctic Biology, is applying his understanding of how muscle cells and metabolism change as we age. His lab has developed a meal replacement powder that can be stirred into liquids and contains a mixture of essential amino acids that support muscle cells. With the meal replacement, older adults wouldn’t have to eat as many sources of whole protein, like meat or beans, to meet their protein needs. This is helpful because these foods are often not pure protein; they contain additional calories from fats and carbohydrates.
The initial clinical trial for this powder, conducted in collaboration with the University of Washington Institute for Translational Health Sciences, has begun recruiting participants. It’s the first NIH-funded clinical trial at the University of Alaska Fairbanks. If successful, the trial would provide evidence that ingesting the meal replacement’s essential amino acids in place of whole protein enables older adults to maintain muscle, lose excess fat, and potentially improve their overall health.
Dr. Rollins’ research is supported by the Institutional Development Award (IDeA) program through NIGMS grant P20GM104318. Dr. Rogers’ research is supported by NIH grant R01AG062575. Dr. Coker’s research is supported by the IDeA program through NIGMS grant P20GM130443.

