 ACTIV clinical trials will evaluate the safety and efficacy of COVID-19 treatments and vaccines. Credit: iStock.
ACTIV clinical trials will evaluate the safety and efficacy of COVID-19 treatments and vaccines. Credit: iStock.
Since the virus that causes COVID-19, known as SARS-CoV-2, was first reported in late 2019, scientists have launched hundreds of studies on strategies for diagnosis, prevention, and treatment. To prioritize the most promising vaccine and therapeutics candidates, streamline clinical trials, and coordinate regulatory processes, the National Institutes of Health (NIH) and the Foundation for the NIH have established the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership. ACTIV brings together eight government entities, 20 biopharmaceutical companies, and four nonprofit organizations.
The public-private partnership provides infrastructure, subject matter expertise, and funding to efficiently bring the most promising therapeutics and vaccines into clinical trials. Five ACTIV therapeutic trials are underway. NIGMS-supported Institutional Development Award Program Infrastructure for Clinical and Translational Research (IDeA-CTR) networks reach historically underserved areas and populations, which are important participants in such trials.
Reaching Rural Residents
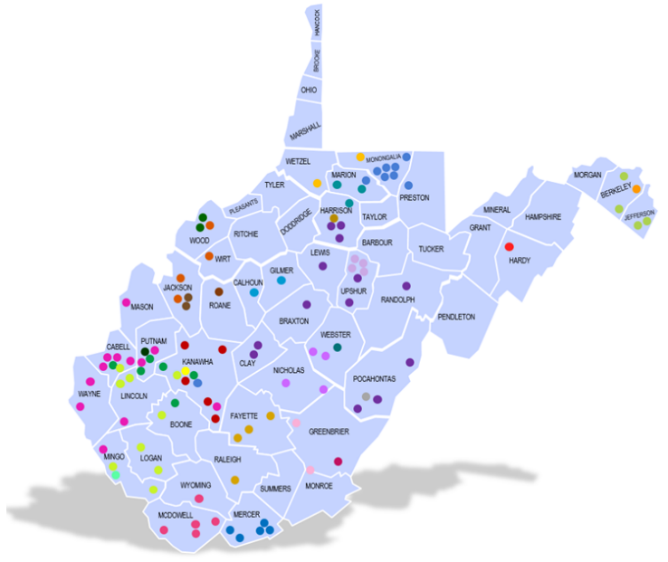 Each dot on this map of West Virginia represents a primary care office or a research entity within the practice-based research network. Credit: West Virginia Clinical and Translational Science Institute.
Each dot on this map of West Virginia represents a primary care office or a research entity within the practice-based research network. Credit: West Virginia Clinical and Translational Science Institute.
While several IDeA-CTR networks are joining one or more ACTIV trials, the West Virginia Clinical and Translational Science Institute and its partners have applied to participate in all of them. The ACTIV 3 trial, which tests the effects of neutralizing antibodies and antivirals in hospitalized patients, has been open since October, and others are expected to start soon.
One goal of the West Virginia Clinical and Translational Science Institute is to make clinical trials available to people living in rural areas. These residents rarely have the chance to participate because such studies are most often conducted at universities and hospitals in urban centers. For at least one of the ACTIV trials, the West Virginia Clinical and Translational Science Institute will enroll participants through some of its practice-based research network sites. The network is comprised of 107 doctors’ offices and research entities throughout the state.
 Dr. Sally Hodder. Credit: West Virginia University.
Dr. Sally Hodder. Credit: West Virginia University.
“It’s so important to make cutting-edge trials available to rural populations,” says Sally Hodder, M.D., a professor of medicine at West Virginia University in Morgantown, associate vice president for Clinical and Translational Science, and director of the West Virginia Clinical and Translational Science Institute. “When folks participate in the science, when there is good community discussion about the trial designs and the results, then I think those populations may be more trusting of the results.” The West Virginia Clinical and Translational Science Institute has an active communications arm, with plans to stay in touch with trial participants and clearly explain trial results once they’re available.
Dr. Hodder hopes that by participating in the ACTIV partnership, the institute will grow its capacity to conduct clinical trials throughout the state and play a meaningful role in combating the COVID-19 pandemic. “We’ve always focused on emerging epidemics, usually in the context of substance use,” she says. “We never imagined emerging epidemics would include the COVID-19 pandemic, but we were really well poised to jump on that.”
For other Biomedical Beat posts and information related to NIGMS’ COVID-19 response, visit our COVID-19 news webpage.
The West Virginia Clinical and Translational Science Institute is supported by the IDeA program through NIGMS grant 5U54GM104942.

