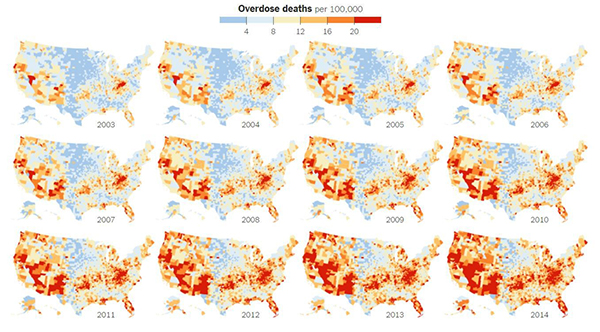
NIGMS cares deeply about our future generations of scientists. That’s why we continue to fund educational tools that make science exciting for students with the hope of steering them toward career paths in science. These materials are available to educators for free through the Science Education Partnership Award (SEPA) program.
SEPA funds innovative Science, Technology, Engineering, and Mathematics (STEM ) and Informal Science Education (ISE ) projects for pre-kindergarten through grade 12. By encouraging interactive partnerships between biomedical and clinical researchers and educators, schools, and other interested organizations, SEPA provides opportunities to:
- Motivate students from underserved communities to consider careers in basic or clinical research
- Improve community health literacy
SEPA-Funded Resources
Here are just a few SEPA-funded resources that educators can use to peak their students’ interest in science:
Charles Darwin Synthetic Interview (middle school through grade 9, and general public)

Credit: The Partnership in Education.
In this free interactive experience for iOS and Android devices, students learn about Charles Darwin, the naturalist, geologist, and leading contributor to the fundamental principles of evolution. Students select from a list of questions to ask a virtual Darwin and receive insight into topics that include:
- His childhood and personal quirks
- His adventures
- Principles of evolution
- Public response to his discovery
Modern-day biologists and other experts provide commentary and answer questions beyond Darwin’s 19th century knowledge. A pay version of the app includes many more questions and answers. Lesson plans and other lessons on evolution are also included with the apps, which were developed by The Partnership in Education at Duquesne University, along with several other SEPA-funded resources.
Continue reading “Spark Student Interest in Science with SEPA-Funded Education Materials”