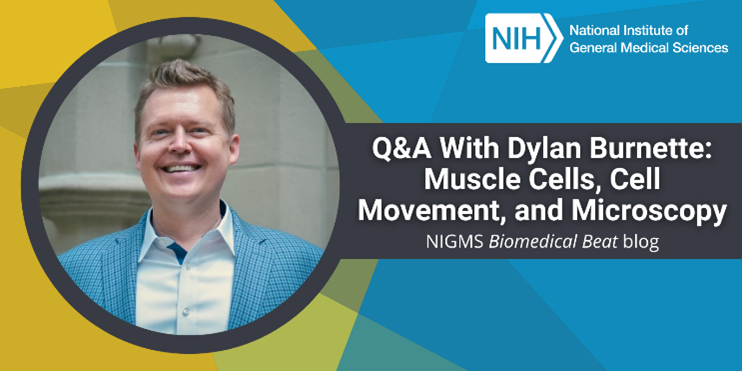
“We scientists know very little of what can be known—I find that invigorating,” says Dylan Burnette, Ph.D., an associate professor of cell and developmental biology at Vanderbilt University School of Medicine in Nashville, Tennessee. “Most people find it exhausting, but I’m comfortable with not knowing all of biology’s secrets.” In an interview, Dr. Burnette shared his lab’s work on muscle cells, the knowledge he hopes readers take away from his research, and some advice to future scientists about being comfortable being wrong.
Q: How did you first become interested in science?
A: Unlike with other subjects (it took me a long time to learn how to read), I excelled at science. In third-grade science class, I knew every answer on the tests. It didn’t occur to me at the time, but I did well because I found it interesting. I decided I wanted to become a medical doctor that year. Back then, doctors were the only type of person who I thought did any type of science.
Continue reading “Q&A With Dylan Burnette: Muscle Cells, Cell Movement, and Microscopy”