What do these images of football fans and bacterial cells have in common? By following simple rules, each individual allows the group to accomplish tasks none of them could do alone—a stadium wave that ripples through the crowd or a cell colony that rebounds after antibiotic treatment.
These collective behaviors are just a few examples of what scientists call emergent phenomena. While the reasons for the emergence of such behavior in groups of birds, fish, ants and other creatures is well understood, they’ve been less clear in bacteria. Two independent research teams have now identified some of the rules bacterial cells follow to enable the colony to persist.
Like pancake batter dropping onto a warm griddle, a bacterial colony grows larger by expanding its outer edge. As it does this, the cells near the periphery have access to nutrients needed for growth but are also exposed to substances that could kill them. Meanwhile, the interior cells are starved, but safe.
“As the colony grows, the inside cells have less access to nutrients and should become dormant or even die, but this is not the case,” explains Darren Sledjeski, one of NIGMS’ experts on bacteria. The inner cells provide a reservoir of living cells that will allow the colony to rebound after the outer cells are harmed by antibiotic treatment or another assault.
How do the cells balance the need to compete for resources and cooperate for survival? It turns out that the inner and outer cells in the colony follow the same rule: restrict nutrients. A research team led by Gürol Süel ![]() of the University of California, San Diego (UCSD), discovered this by studying the growth of Bacillus subtilis biofilms. Biofilms, which contain millions of bacterial cells, are highly resistant to chemicals. For this reason, they can lead to hard-to-treat lung, ear and tooth infections, clog medical implants and coat bathtubs and showers with a slimy residue.
of the University of California, San Diego (UCSD), discovered this by studying the growth of Bacillus subtilis biofilms. Biofilms, which contain millions of bacterial cells, are highly resistant to chemicals. For this reason, they can lead to hard-to-treat lung, ear and tooth infections, clog medical implants and coat bathtubs and showers with a slimy residue.
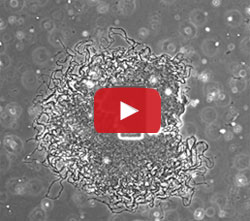
Using a variety of approaches, the UCSD team learned that when the biofilm reached a certain size—about one million cells—the outer cells started consuming all the available glutamate. The starved interior cells then stopped producing ammonium, which halted the growth of the outer cells. As a result of these nutrient-limiting actions, the biofilm growth periodically fluctuated, shown in the image and video above. The results were published last month in Nature. [Editor’s note: A subsequent study by the UCSD team showed that the cells use electrical signaling to coordinate their growth.]
Other types of bacteria may follow different rules to promote the colony’s growth. Instead of restricting resources, E. coli cells seem to share them, according to a March 2015 BMC Systems Biology paper from Zaida Luthey-Schulten and colleagues at the University of Illinois at Urbana-Champaign.
Like human muscle cells, bacteria typically use oxygen to break down glucose into the fuel needed to power cellular processes. But these resources aren’t evenly distributed across a bacterial colony. The Illinois-based studies, which used computational and experimental approaches, showed that as oxygen became less available to the interior cells, these cells broke down glucose into acetate. The acetate became the fuel source for cells with access to oxygen but not glucose. The cells’ cooperation allowed them to persist when the number of cells in the colony increased and resources became more limited.
“As both of these studies show, we’re now starting to get a deeper understanding of the simple rules that bacterial cells follow to drive their collective behavior,” says Sledjeski. “Fundamental insights like these eventually can lead to new strategies for controlling the growth of disease-causing bacteria.”


Excellent strategy unravelled