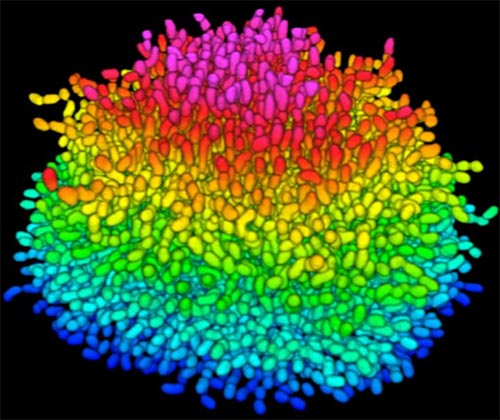
Bacteria use many methods to overcome threats in their environment. One of these ways is forming colonies called biofilms on surfaces of objects. Often appearing like the bubble-shaped fortress represented in this image, biofilms enable bacteria to withstand attacks, compete for space and survive fluctuations in nutrient supply. Bacteria aggregated within biofilms inside our bodies, for example, resist antibiotic therapy more effectively than free swimming cells, making infections difficult to treat. On the other hand, biofilms are also useful for making microbial fuel cells and for waste-water treatment. Learning how biofilms work, therefore, could provide essential tools in our ongoing battle against disease-causing agents and in our efforts to harness beneficial bacterial behaviors. Researchers are now using new imaging techniques to watch how biofilms grow, cell by cell, and to identify more effective ways of disrupting or fostering them.
Until now, poor imaging resolution meant that scientists could not see what individual bacteria in the films are up to as the biofilms grow. The issue is that bacteria are tiny, making imaging each cell, as well as the ability to distinguish each cell in the biofilm community, problematic.
To look inside the biofilm shown here, Jing Yan ![]() and colleagues in the departments of Mechanical and Aerospace Engineering, Molecular Biology and Physics at Princeton University, modified a virulent strain of Vibrio cholerae—the comma-shaped bacterium that causes the disease cholera—so that it glows under certain wavelengths of light. This strategy, coupled with their home-built microscope that is optimized for imaging tiny objects, allowed the researchers to track individual cells as the biofilm formed. They then took hundreds of pictures of a growing biofilm with their custom confocal microscope, which is capable of focusing sharply on one specific region of a specimen at a time. Combining the images with software algorithms that they developed to distinguish each cell enabled the researchers to analyze how the V. cholerae cells were oriented relative to one another as they grew in the film. The scientists could also watch the effects of eliminating single genes that encode proteins V. cholerae cells use to stick to nearby cells and to the surface beneath the biofilm.
and colleagues in the departments of Mechanical and Aerospace Engineering, Molecular Biology and Physics at Princeton University, modified a virulent strain of Vibrio cholerae—the comma-shaped bacterium that causes the disease cholera—so that it glows under certain wavelengths of light. This strategy, coupled with their home-built microscope that is optimized for imaging tiny objects, allowed the researchers to track individual cells as the biofilm formed. They then took hundreds of pictures of a growing biofilm with their custom confocal microscope, which is capable of focusing sharply on one specific region of a specimen at a time. Combining the images with software algorithms that they developed to distinguish each cell enabled the researchers to analyze how the V. cholerae cells were oriented relative to one another as they grew in the film. The scientists could also watch the effects of eliminating single genes that encode proteins V. cholerae cells use to stick to nearby cells and to the surface beneath the biofilm.
The researchers showed that as V. cholerae grows, it develops tightly packed colonies in which cells are brought close together by mechanical forces like compression and are bound by proteins the cells themselves secrete. The scientists observed that when a single, rod-shaped V. cholerae cell rests on a surface, it begins dividing from its narrow ends, and a small, two-dimensional colony initially forms on the surface. Proteins the bacteria secrete keep each cell stuck to the surface and to its neighbor cells. When the colony reaches a certain size, however, the force of the cells on the outer rim pressing on the cells in the center causes the interior cells to break most of their contacts with the surface and flip up perpendicular to it. Since the cells continue to divide from their ends, the center cells force the colony into three dimensions extending out into space. Thus, the bacteria form a dense, organized structure that helps the bacteria residing in the biofilm resist damage from forces like shaking as well as from invading cells.
The scientists also learned that the protein RbmA—previously discovered and characterized by another research team at the University of California, Santa Cruz—plays an important role in the biofilm development process. This protein glues the V. cholerae cells to one another, which ensures that the biofilm structure remains tightly packed as it extends outward. When the researchers made V. cholerae mutants that lacked RbmA, the resulting biofilms were loosely packed and weaker than their wild-type counterparts. This result suggests that structural proteins like RbmA could prove useful as targets for pharmaceuticals that break up biofilms.
The group plans to use its imaging technique to make comparisons with other bacteria that form biofilms. They also aim to describe what happens to cells when the biofilm is exposed to antibiotics or deprived of nutrients. The work could reveal new treatment avenues for manipulating bacteria in biofilms.
This work was funded in part by NIH under grant 5R01GM065859.
More Biomedical Beat Stories




Thank you for giving me a new look at the images in biofilm. I am a new bio-filmmaker who has stepped into film making in this area to read your essay to give me a lot of knowledge.
Wish you more success in the future