It’s back-to-school time. That means learning lots of new facts and figures. In science, terms tend to be several syllables, sometimes with a Latin word thrown into the mix. As a result, many are referred to by their acronyms, such as DNA—short for deoxyribonucleic acid. This makes them easier both to remember and to say.
Researcher Mark Howarth ![]() of Oxford University, has taken this a step further. Searching through information stored in the NIGMS-funded Protein Data Bank
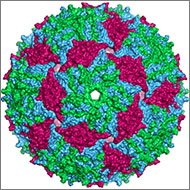
of Oxford University, has taken this a step further. Searching through information stored in the NIGMS-funded Protein Data Bank ![]() , he curated a 3-D protein alphabet. It’s a set of 26 protein crystal structures that look like they were fashioned from bits of rainbow-colored curly ribbon. This 3-D alphabet helps us see what different protein strands look like, and explains terms and concepts relating to protein structure and function.
, he curated a 3-D protein alphabet. It’s a set of 26 protein crystal structures that look like they were fashioned from bits of rainbow-colored curly ribbon. This 3-D alphabet helps us see what different protein strands look like, and explains terms and concepts relating to protein structure and function.
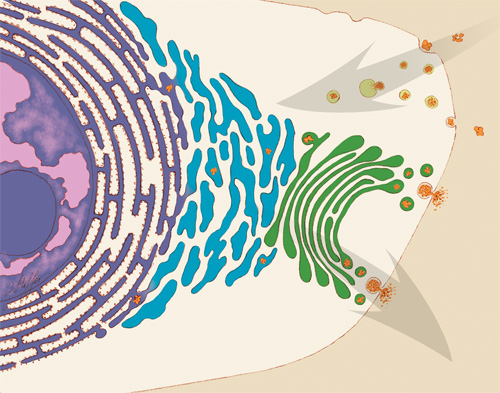
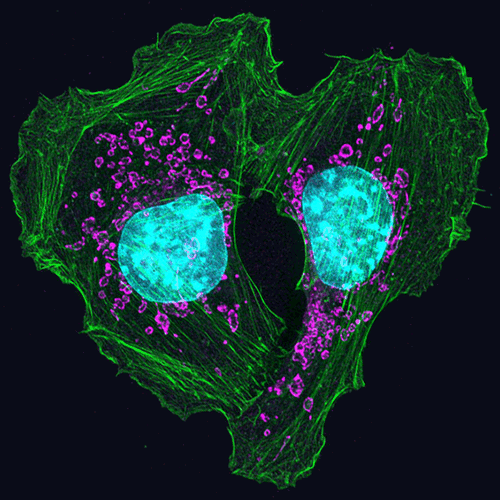
Proteins are molecules that play important roles in virtually every activity in the body. They form hair and fingernails, carry oxygen in the blood, enable muscle movement and much more. Although proteins are made of long strands of small molecules called amino acids, they do not remain as a straight chain. Some proteins are composed of multiple amino acid strands that wind together in the completed protein. The strands twist, bend and fold into a specific shape, and the protein’s structure enables it to perform its task. For instance, the “Y” shape of antibodies helps these immune system proteins bind to foreign molecules such as bacteria or viruses while also drawing in other immune system molecules.
Continue reading “Protein Alphabet: A Picture Is Worth One Letter”