 A cone snail shell. Credit: Kerry Matz, University of Utah.
A cone snail shell. Credit: Kerry Matz, University of Utah.
Over the years, scientists have discovered many compounds in nature that have led to the development of medications. For instance, the molecular structure for aspirin came from willow tree bark, and penicillin was found in a type of mold. And uses of natural products aren’t limited to medicine cabinet staples and antibiotics. A cancer drug was originally found in the bark of the Pacific yew tree, and a medication for chronic pain relief was first isolated from cone snail venom. Today, NIGMS supports scientists in the earliest stages of investigating natural products made by plants, fungi, bacteria, and animals. The results could inform future research and bring advances to the field of medicine.
Seaweed Empowers Neuroscience Research
 Red seaweed is the original source of kainic acid. Credit: Toshiaki Teruya, University of Ryukyus, Japan.
Red seaweed is the original source of kainic acid. Credit: Toshiaki Teruya, University of Ryukyus, Japan.
Helpful natural products have originated in a vast range of environments, from a golf course in Texas to the forests of India. One place researchers continue to explore because of its rich biodiversity is the ocean. Bradley Moore, Ph.D., a professor of pharmacy and pharmaceutical sciences and a member of the Scripps Institution of Oceanography at the University of California, San Diego, studies how marine microbes produce antibiotics, anticancer agents, and other natural products. One of his areas of focus is developing new tools and approaches to identify genes that code for natural products and learning how these products are assembled.
Recently, Dr. Moore discovered a faster and more environmentally friendly way to synthesize kainic acid—a compound produced by some types of red seaweed—that’s valuable for neuroscience research. During a global shortage of this compound in 2000, researchers developed more than 70 chemical synthetic versions. However, the processes for making these synthetic versions have a minimum of six steps and low production rates. Plus, they’re expensive. Dr. Moore’s lab set out to identify the genes that code for the production of kainic acid in red seaweed and found that these genes enabled the seaweed to produce kainic acid in only two steps. By inserting the genes into bacteria, the researchers found they were able to produce gram quantities of kainic acid. This discovery could enable faster and more affordable manufacturing of kainic acid in the future.
Discovering New Antibiotics
Many antibiotics originate from natural compounds that some bacteria produce to protect themselves from other bacteria, and researchers grow a variety of bacteria in the lab to search for new, helpful natural products. But recent discoveries have shown that these bacteria are often capable of producing a greater variety of natural products, including potential antibiotics, than they do under laboratory conditions. In addition, for every bacterial species that grows easily in the lab, there are about a hundred that are difficult or impossible to grow there.
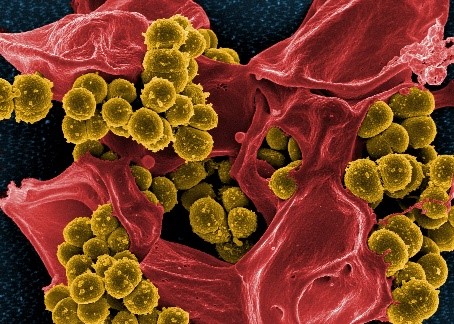 Antibiotic-resistant Staphylococcus aureus bacteria (yellow) and a dead human white blood cell (red).
Antibiotic-resistant Staphylococcus aureus bacteria (yellow) and a dead human white blood cell (red).
Sean F. Brady, Ph.D., tri-institutional professor at Rockefeller University in New York, New York, is developing methods to assess the natural products that bacteria, particularly bacteria in soil, create outside of the lab. He has found a way to extract the DNA that codes for these natural products from bacteria in soil and insert it into bacteria that grow well under lab conditions. This enables researchers to study many natural products that they couldn’t before. Dr. Brady hopes the new products discovered through this process will include some that can function as antibiotics and help address the current problem of antibiotic-resistant bacteria. His lab has already found a number of new antibiotics, including a class called malacidins, from soil bacteria using these methods. Early evidence suggests that malacidins could be effective in treating some antibiotic-resistant infections.
Advancing Natural Products Research with Better Libraries of Microbes
Researchers often look for new, useful natural products in microbial strain libraries, collections of microorganisms with the potential to generate natural products. However, the way scientists create these libraries is costly. In addition, the libraries sometimes have many similar microbes and, thus, many similar or identical natural products. A greater diversity of microbes would be helpful to researchers, giving them a larger range of natural products.
Brian T. Murphy, Ph.D., associate professor of pharmaceutical sciences, and Laura Sanchez, Ph.D., assistant professor of pharmaceutical sciences, both at the University of Illinois at Chicago, developed a way to decrease redundancy in microbial strain libraries and created a web-based tool to help other researchers do the same. Their new method enables them to quickly gather two types of data from microbial colonies: genus and species data, and data on the types of natural products different microbes of the same species produce. Having this data allows them to remove any redundant samples and reduces the cost and effort required to search the libraries later.
Replacing Plastic
The diversity of natural products means they can be used for more than just medications. Some bacteria can produce compounds called polyhydroxyalkanoates (PHAs) that can be used in certain medical applications as biodegradable alternatives to petroleum-based plastic. For instance, there are PHA-based stitches that dissolve harmlessly on their own and don’t require a doctor to remove them. However, producing PHAs is expensive, and this limits their development and commercialization.
Ping Li, Ph.D. , an associate professor of chemistry at Kansas State University in Manhattan, Kansas, is researching how bacteria create PHAs in efforts to produce the compound more economically. His lab focuses on understanding and manipulating proteins and enzymes, and his team is currently investigating the roles and structures of two proteins that are crucial to PHA production and influence PHA’s molecular weight, an important variable when it’s used as a raw material. If scientists can produce PHAs more affordably, the compounds could replace petroleum-based plastic normally used in applications such as tissue engineering, drug delivery, and wound dressing, helping both patients and the environment.
Dr. Moore’s research is supported by NIGMS grant R01GM085770. Drs. Murphy and Sanchez’s research is supported by R01GM125943. Dr. Brady’s research is supported by U01GM110714 and R35GM122559. Dr. Li’s research is supported by R01GM117259.

This post is a great supplement to Pathways: The Superbugs Issue.
Natural products are a source for new antibiotics that may help us fight antibiotic-resistant bacteria and superbugs.
Learn more in our Educator’s Corner.

