Credit: Denise Applewhite.
Grew up in: Princeton, New Jersey
Job site: Columbia University, New York City
Favorite food: Dark chocolate and dark leafy greens
Favorite music: 1940’s style big band jazz
Favorite hobby: Swing dancing
If I weren’t a scientist I would be a: Chocolatier (see “Experiments in Chocolate” sidebar at bottom of story)
One day last fall, molecular biologist Laura Landweber surveyed the Princeton University lab where she’d worked for 22 years. She and her team members had spent many hours that day laboriously affixing yellow Post-it notes to the laboratory equipment—microscopes, centrifuges, computers—they would bring with them to Columbia University, where Landweber had just been appointed full professor. Each Post-it specified the machinery’s location in the new lab. Items that would be left behind—glassware, chemical solutions, furniture, office supplies—were left unlabeled.
As Landweber viewed the lab, decorated with a field of sunny squares, her thoughts turned to another sorting process—the one used by her primary research subject, a microscopic organism, to sift through excess DNA following mating. Rather than using Post-it notes, the creature, a type of single-celled organism called a ciliate, uses small pieces of RNA to tag which bits of genetic material to keep and which to toss.
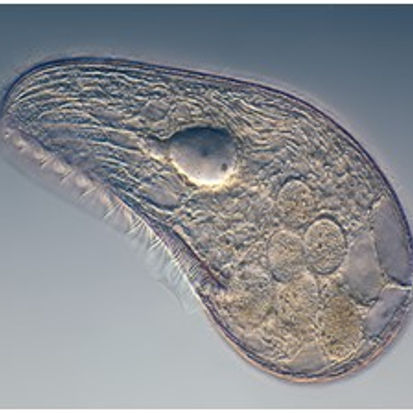
Landweber is particularly fond of Oxytricha trifallax, a ciliate with relatives that live in soil, ponds and oceans all over the world. The kidney-shaped cell is covered with hair-like projections called cilia that help it move around and devour bacteria and algae. Oxytricha is not only bizarre in appearance, it’s also genetically creative.
Unlike humans, whose cells are programmed to die rather than pass on genomic errors, Oxytricha cells appear to delight in genomic chaos. During sexual reproduction, the ciliate shatters the DNA in one of its two nuclei into hundreds of thousands of pieces, descrambles the DNA letters, throws most away, then recombines the rest to create a new genome.
Landweber has set out to understand how—and possibly why—Oxytricha performs these unusual genomic acrobatics. Ultimately, she hopes that learning how Oxytricha rearranges its genome can illuminate some of the events that go awry during cancer, a disease in which the genome often suffers significant reorganization and damage.
Oxytricha’s Unique Features
Oxytricha carries two separate nuclei—a macronucleus and a micronucleus. The macronucleus, by far the larger of the two, functions like a typical genome, the source of gene transcription for proteins. The tiny micronucleus only sees action occasionally, when Oxytricha reproduces sexually.
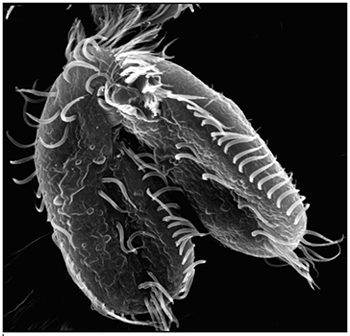
What really makes Oxytricha stand out is what it does with its DNA during the rare occasions that it has sex. When food is readily available, Oxytricha procreates without a partner, like a plant grown from a cutting. But when food is scarce, or the cell is stressed, it seeks a mate. When two Oxytricha cells mate, the micronuclear genomes in each cell swap DNA, then replicate. One copy of the new hybrid micronucleus remains intact, while the other breaks its DNA into hundreds of thousands of pieces, some of which are tagged, recombined, then copied another thousand-fold to form a new macronucleus.
Following up on the work of earlier researchers who provided the first glimpse into the unique nature of sexual development in Oxytricha, Landweber’s lab sequenced the complete genomes of Oxytricha’s two separate nuclei, figured out how the organism tags its tiny pieces of DNA, and deciphered how this marked DNA is guided to the right spot in the new macronucleus.
Oxytricha is not the only unusual research organism Landweber has worked with over the years. Below is a sampling of some of the exotic, and more mainstream, organisms to have spent time under her microscope.
Click on an image below to launch slideshow.
Biology, but also math
Growing up in Princeton, New Jersey, as the daughter of academics, Landweber says her career trajectory almost felt like a foregone conclusion. Early on, her parents fostered a fondness for science, subscribing her to mail order science kits and giving her a microscope for her birthday. In particular, she recalls getting excited about biology around age 10 thanks to a human anatomy coloring book.
Landweber says that, while her “gut instinct” drew her to biology, she also “always loved the mathematical side of things.” She has explored many different ways to combine the two disciplines and often uses math and computer analogies to explain complex biological concepts.
Landweber stayed close to home for college, receiving a degree in molecular biology from Princeton. She then went to graduate school at Harvard University, where her research centered on how errors in RNA are corrected before the molecule is translated into proteins. She likens this process, called RNA editing, to correcting text using a word processor. “Imagine you’re editing a text document and you find a typo,” says Landweber. “You can just go in and fix it before printing.”

After her time at Harvard ended, Landweber returned to her roots, landing a faculty position at Princeton at the remarkably young age of 26. Initially, one research project focused on building computers based on RNA and DNA. For a few years, her lab even held the record for the most powerful molecular computer. But she abandoned the field before too long, convinced that it had no future as a serious competitor to silicon-based computers.
Ciliate Sex
The next field Landweber latched onto, and the one which dominates her current research, was ciliate genomics. She first learned about Oxytricha’s genomic anomalies from a talk she attended by biologist David Prescott, who initially discovered the ciliate’s tiny chromosomes. Right away, Landweber was hooked. “It seemed to fit right into my interest in biological computation, because, when you think about it, ciliates—which scramble, shuffle and reassemble their genes—really are cellular computers,” she says.
Like any typical nucleus, Oxytricha’s macronucleus contains all the DNA it uses to make the RNA and proteins required for everyday life. But the way the DNA in the macronucleus is organized is anything but typical. Humans have 23 pairs of chromosomes. In 2013, Landweber’s team discovered that Oxytricha’s macronucleus contains roughly 16,000 unique chromosomes—nearly 700 times more than we have!
Oxytricha’s micronucleus supplies the pool, or reservoir, of genetic material that it can exchange with another Oxytricha cell. During mating, the micronuclei provide all the drama—they divide by meiosis, discard unneeded material, exchange DNA with the partner cell, shatter into thousands of pieces, shuffle and scramble their DNA. Meanwhile, the macronuclei completely degrade.
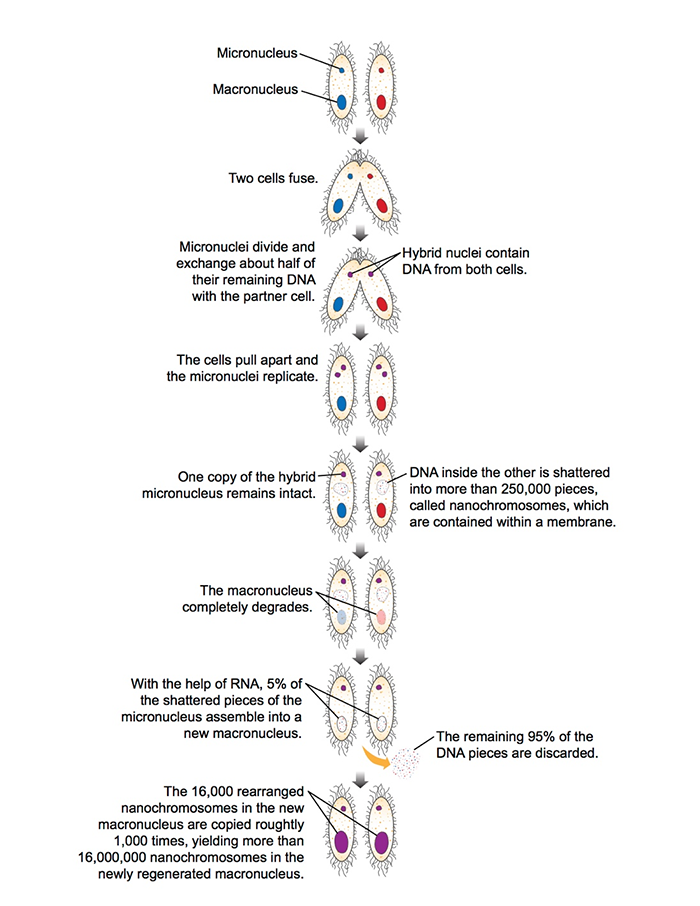
The entire process is quite complicated, but here’s the basic idea (see illustration for a simplified version). Two Oxytricha cells fuse, creating a connecting structure that bridges the insides of both cells. About one half of the DNA in each cell’s micronucleus travels across this bridge into the partner cell. In each cell, the fresh DNA combines with existing DNA to create a hybrid micronucleus.
The two cells split apart, and the hybrid micronucleus in each makes a copy of itself. In each cell, one of the two new micronuclei remains intact, while the DNA in the other one breaks apart into nearly 250,000 disordered pieces.
Then, Oxytricha spends two days discarding about 95 percent of the DNA from shattered micronucleus. The remaining 5 percent of its DNA rearranges (sometimes scrambled or turned upside down), and reassembles to construct the creature’s new macronucleus.
At this point, the macronucleus contains about 16,000 different nanochromosomes—some of which are just a single gene in length, or about 3,000 letters. These nanochromosomes are then amplified another thousand-fold, yielding about 16 million nanochromosomes in the completed macronucleus, which explains its larger size.
At the end of the process, which can take three or four days, the micronucleus and macronucleus of both Oxytricha cells are genetically rejuvenated, incorporating DNA from each cell’s original cache as well as new bits from its partner.
Landweber digs deeper into Oxytricha genomics
While Landweber and her team initially thought the discarded, or leftover, DNA of the shattered micronucleus was “junk,” they later discovered that a substantial chunk of this DNA encodes enzymes called transposases, which are partly responsible for shuffling and reordering the genes of the micronucleus and stitching them together into the new macronucleus.
Landweber and her colleagues subsequently learned a few additional details about the process that harken back to her early interest in RNA. They discovered that small, non-coding RNAs called piRNAs play the role of Post-it notes, flagging the 5 percent of micronucleus DNA to keep for Oxytricha’s macronucleus. In addition, Landweber’s team discovered that long, non-coding RNAs help steer the tagged DNA to the right location in the remodeled macronucleus.
Given the rather convoluted nature of Oxytricha sex, one question Landweber is often asked is “Why does such a complicated system exist?” Landweber offers a few possibilities. First, encrypting its DNA may protect Oxytricha against viruses that attempt to take up residence in its genome. Second, the ability to come up with new combinations of DNA can be used to create new genes, but this happens rarely enough that she thinks it’s something of a side benefit. Instead, Landweber leans more toward the idea that the system evolved as an error-correction mechanism that relies on RNA to ensure the precise transfer of genetic information across generations.
No matter what the ultimate reason turns out to be, Landweber continues to be enthralled with Oxytricha. “It is just truly marvelous that nature has invented such a clever mechanism,” she says.
Bringing Oxytricha to the clinic
While Oxytricha can provide insights to the burgeoning field of genome editing, particularly relevant to human health is the observation that so many human cancers are defined by fairly drastic chromosome reorganization. One extreme example is chromothripsis—a rare occurrence linked to a variety of cancers, in which a single catastrophic event triggers thousands of chromosomal rearrangements.
“Oxytricha can teach us a lot about the possibilities of maintaining genome integrity in the face of destructive forces that can corrupt it, including cancer,” says Landweber.
Landweber hopes that her lab’s discoveries in Oxytricha will help improve our understanding of events that can lead to cancer. “It’s as though Oxytricha’s process of rebuilding its chromosomes runs the clock in reverse, starting from a shattered genome and restoring integrity,” she says. “We need to tap into that.”
Within a short walk of her new lab overlooking the Hudson River at Columbia University Medical Center, Landweber has found many potential clinical collaborators. “I’m now actually surrounded by a lot of people studying problems of cancer biology,” she says.
As Landweber prepares to apply her knowledge of ciliate biology to combating cancer, she plans to continue seeking to understand exactly how Oxytricha can manage the chromosomal chaos that would be catastrophic to human cells.

Like Landweber herself, Landweber’s three daughters with husband Steven Gubser, a theoretical physicist at Princeton, are growing up in a household steeped in academia. However, while conversations around the dinner table might revolve around science, Landweber says her and Steven’s best collaborations occur in the kitchen.
“That’s where he can be an experimentalist,” Landweber adds, “And we absolutely do like to experiment with making things like what we claim to be healthy chocolate cakes.”
While the healthiness of her cakes may be in question, Landweber’s bona fides as a chocolate lover are not. Just after graduate school, she served as Chocolate Steward of the Harvard Society of Fellows.
“Among other things, being steward meant that I had a little bit of clout when I’d walk into a chocolate shop,” says Landweber. “I would get a few more tastings and a few more tips, and I learned a lot about chocolate.”
She poured most of what she learned into one of the first online guides to chocolate in Boston, which, to her surprise, was for years the number one page resulting from a Google search of “chocolate” and “Boston.”
Landweber’s research is funded in part by NIGMS grant R01GM059708.