Educators often struggle to teach teens about sexual and reproductive health. Hexacago Health Academy (HHA) ![]() , an education program from the University of Chicago, leverages the fun activity of gameplay to impart these lessons to young people from Chicago’s South Side community. Funded by the Student Education Partnership Award (SEPA)
, an education program from the University of Chicago, leverages the fun activity of gameplay to impart these lessons to young people from Chicago’s South Side community. Funded by the Student Education Partnership Award (SEPA)![]() , part of the National Institute of General Medical Sciences (NIGMS), in 2015, HHA assists teachers in their goal of helping teen students gain awareness and control over their health and also learn about careers in STEM
, part of the National Institute of General Medical Sciences (NIGMS), in 2015, HHA assists teachers in their goal of helping teen students gain awareness and control over their health and also learn about careers in STEM ![]() and health fields.
and health fields.

Genesis of HHA
HHA was cofounded by Melissa Gilliam ![]() , a University of Chicago professor of Obstetrics/Gynecology and Pediatrics and founder of the Center for Interdisciplinary Inquiry & Innovation in Sexual and Reproductive Health (Ci3)
, a University of Chicago professor of Obstetrics/Gynecology and Pediatrics and founder of the Center for Interdisciplinary Inquiry & Innovation in Sexual and Reproductive Health (Ci3) ![]() . During a 2013 summer program with high school students, Gilliam and Patrick Jagoda
. During a 2013 summer program with high school students, Gilliam and Patrick Jagoda ![]() , associate professor of English and Cinema & Media Studies, and cofounder of Ci3’s Game Changer Chicago Design Lab, introduced the students to their STEM-based alternate reality game called The Source, in which a young woman crowdsources player help to solve a mystery that her father has created for her.
, associate professor of English and Cinema & Media Studies, and cofounder of Ci3’s Game Changer Chicago Design Lab, introduced the students to their STEM-based alternate reality game called The Source, in which a young woman crowdsources player help to solve a mystery that her father has created for her.
From their experience with The Source, Gilliam and Jagoda quickly learned that students not only wanted to play games but to design them too. What followed was the Game Changer Lab’s creation of the Hexacago game board, as well as the launch of HHA, a SEPA-funded project that the lab oversees.
Hexacago Game Board
At the core of HHA is the Hexacago game board, which displays the city of Chicago, along with Lake Michigan, a train line running through the city, and neighborhoods gridded into a hexagonal pattern.
HHA students not only play games designed from the Hexacago board template, but also design their own games from it that are intended to inspire behavior change in health-related situations and improve academic performance.

In this way, HHA is much more than just game design and play. “Students have no idea that what they’re doing is learning. In their minds, they’re really focused on designing games,” says Gilliam. “That’s the idea behind Hexacago Health Academy: helping people acquire deep knowledge of science and health issues by putting on the hat of a game designer.” Moreover, through the process of gameplay and design, students practice all the rich skills that result from teamwork, including collaborative learning, leadership, and communication.
Continue reading “Teens Explore Science and Health through Game Design”


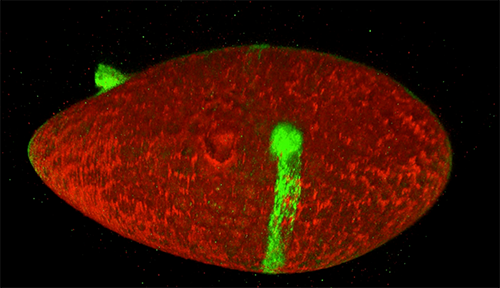
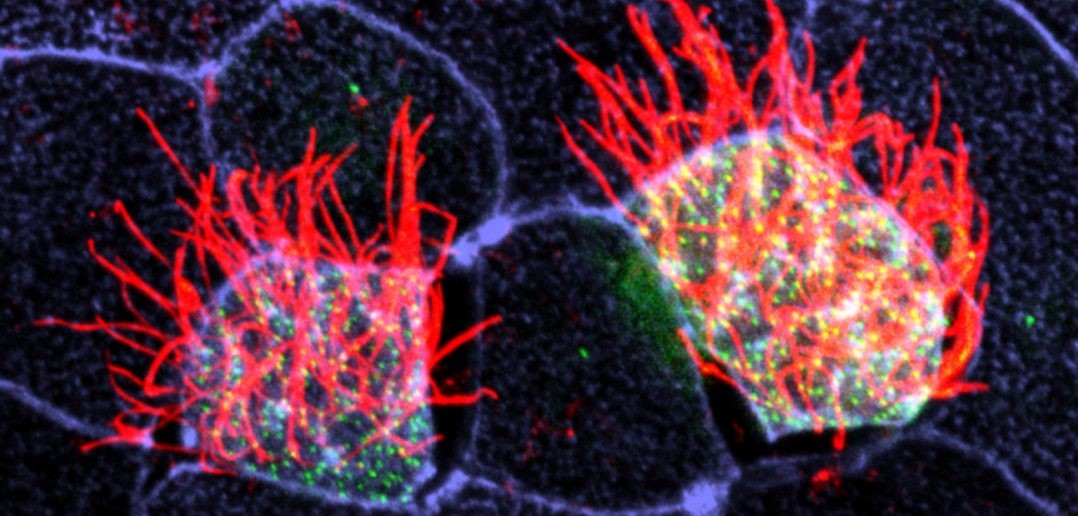 Cells covered with cilia (red strands) on the surface of frog embryos. Credit: The Mitchell Lab.
Cells covered with cilia (red strands) on the surface of frog embryos. Credit: The Mitchell Lab.
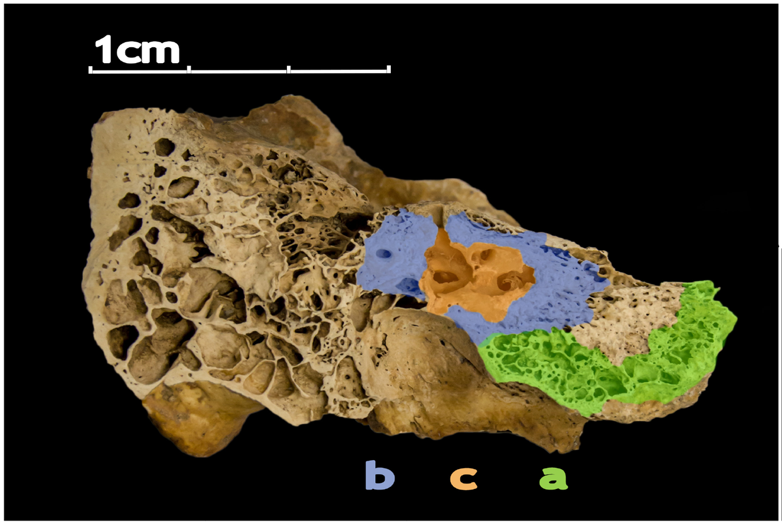 The human petrous bone in the skull protects the inner ear structures. Though it is one of the hardest, densest bones in the body, some portions (such as the area in orange, protecting the cochlea) are denser than others. Possibly because the petrous bone is so dense, DNA within the petrous bone is better preserved than in other bones. In some cases, scientists have extracted more than 100 times more DNA from the petrous bone than other bones, including teeth. Credit:
The human petrous bone in the skull protects the inner ear structures. Though it is one of the hardest, densest bones in the body, some portions (such as the area in orange, protecting the cochlea) are denser than others. Possibly because the petrous bone is so dense, DNA within the petrous bone is better preserved than in other bones. In some cases, scientists have extracted more than 100 times more DNA from the petrous bone than other bones, including teeth. Credit: