“My parents told me that I already wanted to be a scientist when I was 7 or 8 years old. I don’t remember ever considering anything else,” says Ry Young, Ph.D., a professor of biochemistry, biophysics, and biology at Texas A&M University, College Station.
Dr. Young has been a researcher for more than 45 years and is a leading expert on bacteriophages—viruses that infect bacteria. He and other scientists have shown that phages, as bacteriophages are often called, could help us fight bacteria that have developed resistance to antibiotics. Antibiotic-resistant infections cause more than 35,000 deaths per year in the U.S., and new, effective treatments for them are urgently needed.
Starting Out in Science
Dr. Young knew he wanted to study biology from the time he read a children’s book about Louis Pasteur. He earned a bachelor’s degree in biochemistry from Rice University, Houston, Texas, and was about to start a Ph.D. program when he received a draft notice from the military. Dr. Young spent 3 years in the Navy and read biology textbooks in his spare time.
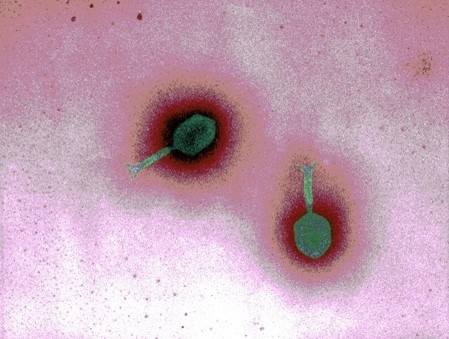
After completing his military service, Dr. Young entered a molecular biology Ph.D. program at the University of Texas at Dallas, where he conducted his doctoral research on the control of bacterial ribosome synthesis. He used phages as tools to move DNA between bacteria, but the viruses weren’t his focus.
Dr. Young went on to work as a postdoc at Harvard Medical School, Boston. There, he studied bacterial transposons, also known as “jumping genes”—sequences of DNA that can change position within the genome. Again, he was using phages as tools, and it led to a discovery that altered the course of his career.
Diving Into the Basic Biology of Bacteriophages
One day in 1976, Dr. Young prepared two flasks of bacterial culture. Both were filled with cloudy-looking liquid, and he added phages that contained a transposon to one and phages that didn’t to the other. After about 45 minutes, he looked down and, to his surprise, saw the liquid in one flask rapidly turn clear while the other remained cloudy. “One whole culture just disappeared in front of my eyes,” he says. The increase in transparency indicated that the bacteria had burst open, a process known as lysis, but it only happened in the liquid containing the phage without the transposon.
Bacterial lysis was long thought to be a passive process that occurred when a bacterial cell could not maintain its internal balance. But Dr. Young’s observations suggested that phages could not only infect bacteria but also intentionally trigger lysis. However, the liquid treated with transposon-containing phages remained cloudy—which Dr. Young realized was because the transposon jumped into a viral gene that helps phages cause bacterial lysis and stopped it from working.

Dr. Young was fascinated by the revelation, but phage-induced bacterial lysis didn’t become the focus of his research until he joined the faculty of Texas A&M in 1978 and received NIGMS funding for his phage work. He ultimately discovered two ways that phages cause bacteria to burst: multigene lysis and single-gene lysis. Multigene lysis, as the name implies, involves more than one of the phage’s genes. It’s the more complex of the two processes, the only one with a molecular clock (meaning the lysis occurs at a precise time and not haphazardly), and what Dr. Young first observed in his experiments with the two culture flasks.
Single-gene lysis requires just one gene, which contains the instructions for a protein that triggers lysis independently. Every one of the thousands of phages that use single-gene lysis makes its own unique protein. “The way I look at it is each protein is pointing to a self-destruct button somewhere in the bacteria,” says Dr. Young. Most currently available antibiotics kill bacteria by interfering with the building of their cell walls, but some bacteria have mutated so the antibiotics are no longer effective. Dr. Young hopes that identifying the bacterial “buttons” will enable other researchers to develop new antibiotics that target them.
The Future of Phages

In addition to his work on phage-induced lysis, Dr. Young has contributed to the development of phage therapies, which use phages themselves to treat bacterial infections. In 2016, he helped create one of two phage mixtures given to a patient infected with antibiotic-resistant Acinetobacter baumanii bacteria under a special protocol for experimental therapies. The patient made a full recovery, becoming the first person in the United States to successfully receive an intravenous phage treatment. Dr. Young and the Center for Phage Technology, which he directs, have since helped treat several more patients.
The treatments have all been last resorts for patients with no other options. When asked what he thinks the future of phage therapies will look like, Dr. Young replies, “I tell my graduate students that ‘I don’t know’ are the three most important words in science.” He says it’s unclear whether phage therapies can be successfully commercialized for several reasons—one of them being the fact that phages are very specific against a single type of bacteria rather than being able to treat a wide variety of bacterial infections. If they are commercialized, his gut instinct is that the treatments will be reserved for infections that antibiotics can’t treat.
Phage therapies have reignited scientific interest in phage biology, which seemed to be a dying field for most of Dr. Young’s career. For the first time in decades, young researchers are starting phage-focused labs. “I think now there’s going to be a renaissance, but we came pretty close to being a field that just expired,” Dr. Young says.
Dr. Young is retiring from teaching in spring 2022 but plans to continue his research. When asked what he’s looking forward to about retirement, he says, “My wife and I are buying a boat, and we’re going to be spending a lot of time on it. But the real fun part is knowing I can get up every morning and go into the laboratory and not think about anything except experiments.”
Dr. Young’s research is supported by NIGMS grant R35GM136396.