According to a recent estimate, implant infections following hip and knee replacement surgeries in the U.S. may number 65,000 by 2020, with the associated healthcare costs exceeding $1 billion. A new small, high-tech device could have a significant impact on improving health outcomes and reducing cost for these types of surgeries. The device, Air Barrier System (ABS), attaches on top of the surgical drape and gently emits HEPA-filtered air over the incision site. By creating a “cocoon” of clean air, the device prevents airborne particles—including the bacteria that can cause healthcare-associated infections—from entering the wound.
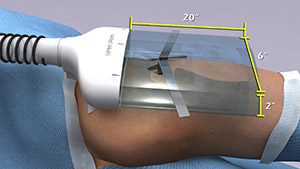
Scientists recently analyzed the effectiveness of the ABS device in a clinical study—funded by NIGMS—involving nearly 300 patients. Each patient needed an implant, such as an artificial hip, a blood vessel graft in the leg or a titanium plate in the spine. Because implant operations involve inserting foreign materials permanently into the body, they present an even higher risk of infection than many other surgeries, and implant infections can cause life-long problems.
The researchers focused on one of the most common causes of implant infections—the air in the operating room. Although operating rooms are much cleaner than almost any other non-hospital setting, it’s nearly impossible to sterilize the entire room. Instead, the scientists focused on reducing contaminants directly over the surgical site. They theorized that if the air around the wound was cleaner, the number of implant infections might go down. Continue reading “New Technology May Help Reduce Serious and Costly Post-Surgical Infections—Using Nothing but Air”